Hierarchical porous carbon materials derived from a waste paper towel with ultrafast and ultrahigh performance for adsorption of tetracycline†
Abstract
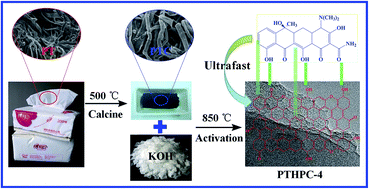
Herein, waste paper towels, a mass garbage product from daily life, were used as precursors for the production of hierarchical porous carbon (PTHPC) via KOH activation to be used for the removal of tetracycline. In this study, the influence of KOH content on porosity and adsorption capacity was investigated, and the optimal property of hierarchical porous carbon was obtained at weight ratios of carbonization: KOH = 1 : 4 (PTHPC-4). The physico-chemical properties of PTHPC-4 were characterized by different technology. Notably, PTHPC-4 possesses an ultrahigh specific surface area of 3524 m2 g−1 and a large pore volume of 1.839 cm3 g−1. Additionally, the isothermal adsorption results showed that PTHPC-4 displayed an ultrahigh adsorption capacity of 1661.13 mg g−1 for tetracycline, which is superior to other previously reported adsorbents. Moreover, PTHPC-4 also has excellent kinetics performance: when C0 = 100 mg L−1, the pseudo-second-order kinetics constant k2 values at 298, 308 and 318 K are 1.055 × 10−2, 5.731 × 10−2 and 8.916 × 10−2 g mg−1 min−1, respectively, which were 1–3 magnitude higher than previously reported adsorbents. In addition, the investigation of the effect temperature, pH and ionic strength on the adsorption property for tetracycline are included in this study.

 Please wait while we load your content...
Please wait while we load your content...