Enhanced metal–support interactions between Pd NPs and ZrSBA-15 for efficient aerobic benzyl alcohol oxidation†
Ran Ji,
Shangru Zhai*,
Wei Zheng,
Zuoyi Xiao*,
Qingda An and
Feng Zhang*
Faculty of Light Industry and Chemical Engineering, Dalian Polytechnic University, Dalian 116034, China. E-mail: zhaisr@dlpue.du.cn; xiaozy@dlpu.edu.cn; zhang_feng@dlpu.edu.cn
First published on 12th July 2016
Abstract
Towards the aerobic oxidation of alcohols, palladium nanoparticle immobilized mesoporous catalysts have been extensively investigated; however, the preparation of efficient catalytic materials without using complexing moieties remains a challenge. Herein, a simple yet effective concept that directly utilizes inorganic ZrSBA-15, a mild acidic framework, as a supporting matrix for Pd NPs was applied. The catalysts were fully characterized using X-ray diffraction, N2 adsorption–desorption isotherms, Fourier-transform infrared spectroscopy, X-ray photoelectron spectroscopy, scanning electron microscopy and transmission electron microscopy. It was demonstrated that, on the basis of these characterization results, active Pd NPs had been successfully loaded onto the porous framework of ordered ZrSBA-15. More significantly, the method of preparation was also attributed to the catalytic properties of the resultant sample, in which the interactions between Pd species and acidic framework were improved, leading to a uniform dispersion and enhanced stabilization of Pd particles. Meanwhile, Pd/ZrSBA-15 catalysts exhibited expected activity in the aerobic oxidation of benzyl alcohol with at least 95% conversion and 99% selectivity for benzaldehyde. Moreover, the oxidation reaction could be performed smoothly under solvent- and base-free conditions, and the catalyst was recycled several times with negligible loss in selectivity and conversion.
1. Introduction
A variety of techniques and technologies have been used to improve the environmental performance of oxidation reactions, e.g. avoiding toxic reagents such as organic peroxides, using solvents such as water or solvent free systems, and using heterogeneous catalysts based on the benefits of easy separation and catalyst recovery. By these means, the reaction selectivity can be improved by controlling the chemistry and structure of solid catalysts.1,2In the past few years, the oxidation of alcohols in industry has mostly been based on the use of stoichiometric amounts of CrO3, K2Cr2O7, KMnO4, MnO2, etc., which are expensive and environmentally unfriendly.3 In particular, the selective oxidation of benzyl alcohol (BnOH) to benzaldehyde (BzH) is a significant reaction for obtaining chlorine-free BzH, essential to perfumery and food industries.4 Eco-efficient oxidation of BnOH can be carried out in the aqueous phase or under solventless conditions and by using molecular oxygen or hydrogen peroxide as oxidant. Previously, the most used catalysts for the oxidation of benzyl alcohol have been metal nanoparticles supported on a variety of materials.5 Supported catalysts such as Au/CeO2,6 Au/TiO2,7 Au/MgO,8 Au/CuO,9 Pd/HT,10 Pd/HAP,11 Pd/Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 and Au–Pd/TiO2
12 and Au–Pd/TiO2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13have been reported exhibiting high catalytic activities in the oxidation of BnOH with atmospheric oxygen and under solventless conditions. However, the preparation process of these catalysts are complicated, and, more unfavorably, complexing agents are a requisite to efficiently anchor and stabilize active metal particles. For instance, during the synthesis of polystyrene/gold–platinum composite particles,14 bimetallic nanoparticles were attached to the surface of the catalyst by the carboxyl groups derived from trisodium citrate. However, considering the fact that the support is not a channel-structured matrix, the space for the active component would have been restricted. More seriously, a significant waste of precious metals in the process of preparation was apparent. Meanwhile, the catalytic properties of the nanoparticles are highly sensitive to the size, morphology, composition, and dispersion of the metal particles.15–17 Within this context, some supports have to be modified by the functional groups to affect the dispersion of noble metals.18 Therefore, the preparation of highly efficient catalysts with enhanced metal–support interaction using a simple, controllable and cost-effective approach remains an issue demanding a prompt solution in catalysis.
13have been reported exhibiting high catalytic activities in the oxidation of BnOH with atmospheric oxygen and under solventless conditions. However, the preparation process of these catalysts are complicated, and, more unfavorably, complexing agents are a requisite to efficiently anchor and stabilize active metal particles. For instance, during the synthesis of polystyrene/gold–platinum composite particles,14 bimetallic nanoparticles were attached to the surface of the catalyst by the carboxyl groups derived from trisodium citrate. However, considering the fact that the support is not a channel-structured matrix, the space for the active component would have been restricted. More seriously, a significant waste of precious metals in the process of preparation was apparent. Meanwhile, the catalytic properties of the nanoparticles are highly sensitive to the size, morphology, composition, and dispersion of the metal particles.15–17 Within this context, some supports have to be modified by the functional groups to affect the dispersion of noble metals.18 Therefore, the preparation of highly efficient catalysts with enhanced metal–support interaction using a simple, controllable and cost-effective approach remains an issue demanding a prompt solution in catalysis.
As a matter of fact, those catalysts prepared by the immobilization of palladium nanoparticles (Pd NPs) have also shown to be catalytically active in the oxidation of BnOH. Numerous methods exist for anchoring Pd NPs in mesoporous silica such as ion exchange, wetness impregnation, chemical vapour infiltration, and in situ reduction.19,20 In general, ultrafine Pd NPs are vulnerable to serious aggregation; to handle this problem, capping agents (surfactants or polymeric stabilizers) are commonly used to stabilize the Pd NPs.21,22 However, in most of the cases, the removal of the capping agent is required to activate the catalyst. This is usually accomplished by washing and/or heating treatment.23
Owing to its highly ordered mesopores, large surface area, relatively large pore sizes, and higher hydrothermal stability, mesoporous silica SBA-15 has become a desirable solid support for Pd NPs.24,25 For example, Gao and co-workers reported a simple one-step method to synthesize Pd NPs in SBA-15 by a sol–gel method, with the prepared catalyst having a pore size in the range of 5–10 nm, a large specific area and a highly ordered pore structure.26 However, the efficiency of the catalyst is slightly too low, due to weak metal–support interaction which causes the problem of leaching of the active species. A Cu(II)-Schiff base complex covalently grafted over 3-aminopropyl functionalized SBA-15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 has shown better catalytic activity in the presence of H2O2 and in the aqueous phase. By contrast, there are only a few reports about the use of Pd NPs loaded on SBA-15 directly in BnOH oxidation, and Pd NPs must be bonded strongly enough to the support to avoid leaching processes.
27 has shown better catalytic activity in the presence of H2O2 and in the aqueous phase. By contrast, there are only a few reports about the use of Pd NPs loaded on SBA-15 directly in BnOH oxidation, and Pd NPs must be bonded strongly enough to the support to avoid leaching processes.
Moreover, to efficiently disperse and stabilize the supported Pd NPs, the morphology of SBA-15 was also tailored in some cases. For example, the preparation of SBA-15 with rod-like,28 platelet-like, and fiber-like29 morphologies, which have been employed as supporting materials for various metal species, have been extensively investigated. Among them, Zr-containing SBA-15 with platelet morphology (as is reported, Zr ions have the ability to accelerate the self-assembly of P123 micelles and the silica source, leading to the formation of greatly shortened meso-channels30,31) is seemingly a suitable support for Pd NPs on account of its ease for molecular diffusion; namely, the significantly shortened channels offer quicker mass transportation and accordingly higher reaction efficiency.32–36 More importantly, the Zr atoms, incorporated into the framework channels and serving as the high charge density sites, not only can improve the hydrothermal stability of the pore walls but also can increase the number of lattice defects in the skeleton. Possibly, by virtue of these features, ZrSBA-15, with increased acidic sites as compared to purely siliceous SBA-15, can favorably enhance the metal–support interactions between Pd NPs and ZrSBA-15.
Herein, in continuation of our previous work designing high-performance oxidation catalysts,37 we present a facile and cost-effective approach to prepare ZrSBA-15 supported Pd catalysts. By this means, Pd NPs could be easily deposited into the interior of ZrSBA-15, with a narrow size distribution between 1.9 and 2.3 nm. The catalytic performance of the resultant samples was evaluated by base-free selective oxidation of BnOH with oxygen as a green oxidant under solvent-free reaction conditions. The reaction parameters were optimized, and the recycling performance was also comparatively studied between Pd/ZrSBA-15 and Pd/SBA-15 with identical loading of active Pd particles. The results demonstrated that this methodology offers a greener alternative for the selective oxidation of BnOH in a solvent- and base-free reaction system.
2. Experimental section
2.1 Materials and chemicals
All reagents and solvents were of analytical grade and were used as received. Polymeric P123 (EO20-PO70-EO20) was purchased from Aldrich. Zirconium oxychloride (ZrOCl2·8H2O), benzyl alcohol, benzaldehyde, nitrobenzene, tetraethyl orthosilicate (TEOS), and concentrated hydrochloric acid (HCl, 37%) were procured from Sinopharm Chemical Reagent Co. Ltd. Benzaldehyde was obtained from the Kermel Company, China. Potassium tetrachloropalladate (K2PdCl4) was purchased from Aladdin and used as received.2.2 Catalyst preparation
2.3 Catalytic oxidation test
The catalytic performance was evaluated by the solvent-free aerobic oxidation of BnOH, which was carried out in a 50 mL three-necked flask with a reflux condenser, under O2 pressure at 1 bar. After a determined time, the samples were rapidly cooled in a cold water bath and the reaction mixture was dissolved in ethyl acetate and was centrifuged to remove the catalyst particles. The reaction products were quantitatively and qualitatively analyzed by GC by using nitrobenzene as the internal standard. The conversion and selectivity were calculated as follows:2.4 Characterization
X-ray diffraction (XRD) patterns were recorded by a Shimadzu XRD-6100 diffractometer with CuKα radiation (λ = 1.54060 Å) from 10–80° at a 5° min−1 scanning speed. N2 adsorption–desorption isotherms were recorded at 77 K with a Quantachrome Autosorb NOVA 2200e analyzer; prior to analysis the samples were degassed at 250 °C under vacuum (1.33 Pa) for 2 h. Specific surface area was calculated by the BET method, and the total pore volume was the volume of liquid nitrogen adsorbed at a relative pressure of 0.99 Pa. The pore size distribution (PSD) and the pore diameter (Dp) were calculated from the desorption branch by using the Barrett–Joyner–Hallenda (BJH) method. Transmission electron microscopy (TEM) images were observed using a Hitachi HT7700 microscope. Scanning electron microscopy (SEM) images were observed using Hitachi S-5500. The metal content of palladium in terms of palladium percentage was determined by a ICP-OES Perkin-Elmer Optima 5300 DV instrument.3. Results and discussion
3.1 Structural characterization of prepared samples
The strategy for immobilizing Pd on the ZrSBA-15 is illustrated in Scheme 1. Noticeably, it is a facile and cost-effective approach to prepare ZrSBA-15 supported Pd catalysts. On one hand, the flakes and short channels of the ZrSBA-15 could have adsorbed PdCl42− ions favorably from an aqueous solution of K2PdCl4. On the other hand, after reduction by freshly prepared NaBH4 solution at room temperature, well dispersed Pd NPs could be easily hosted in the channels of ZrSBA-15.Firstly, XRD techniques were employed to assess the structural ordering of the resultant samples. The small-angle XRD patterns of ZrSBA-15, 3.3 wt% Pd/ZrSBA-15 and 3.3 wt% Pd/ZrSBA-15/5t are shown in Fig. 1a (the “5t” appended to the catalyst name indicates that the catalyst had been recycled five times, see below). Although the position of the diffraction peaks slightly changed from sample to sample, all materials obviously exhibited three well resolved peaks related to the (100), (111), and (200) planes. These diffraction lines were associated with 2D hexagonal ordering in the P6mm space group; these results were consistent with the data reported by Zhao for SBA-15.38,39 Besides, although the intensity of characteristic peaks of 3.3 wt% Pd/ZrSBA-15 and 3.3 wt% Pd/ZrSBA-15/5t decreased slightly as compared with ZrSBA-15, the difference in the position was not significant. This indicated that the hexagonal pore arrangement of the support was not obviously changed during the adsorption–deposition of Pd NPs. Additionally, with the increase of Pd loading, the intensity of the (110) diffraction peaks obviously decreased. This indicates that Pd nanoparticles synthesized on the ZrSBA-15 support are mainly enclosed by (110) facets. This is probably due to the fact that, along with the introduction of the Pd particles, the scattering intensity of amorphous pore walls was reduced. This suggested that Pd NPs were mainly distributed into the channels of the ZrSBA-15 supporting matrix.
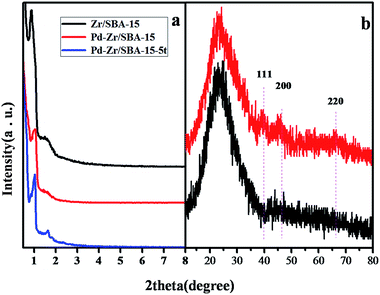 | ||
| Fig. 1 (a) Small-angle XRD patterns of ZrSBA-15, 3.3 wt% Pd/ZrSBA-15 and 3.3 wt% Pd/ZrSBA-15/5t; (b) wide-angle XRD patterns of ZrSBA-15 and 3.3 wt% Pd/ZrSBA-15. | ||
Accordingly, as shown in Fig. 1b, the wide-angle XRD patterns of ZrSBA-15 and 3.3 wt% Pd/ZrSBA-15 showed a typical broad diffraction peak at about 22.44°, corresponding to an amorphous silica phase in nature. Besides this, for 3.3 wt% Pd/ZrSBA-15, the three minor diffraction peaks at 2θ = 40.01°, 46.12° and 66.50° can be ascribed to the diffraction of the (111), (200) and (220) planes of face-centered cubic Pd; also, it reveals that the Pd species remain in metallic state,40 indicating that the Pd species have been highly dispersed into the porous channels of the support.
Following the XRD analysis results, the N2 adsorption–desorption isotherms of ZrSBA-15, 3.3 wt% Pd/ZrSBA-15, 3.3 wt% Pd/ZrSBA-15/5t are shown in Fig. 2. As is reported for SBA-15 related materials,41 not only did the isotherms allow the calculation of specific surface area, pore volume and pore size, but also the position and shape of hysteresis loop could provide useful information about the shape and ordering of the pore arrangements of the resultant composite. Clearly, type IV isotherms with sharp hysteresis loops can be observed at relative pressures of P/P0 = 0.6–0.8, which can be corresponded to the capillary condensation of N2; this also indicates that a well defined cylindrical narrow pore size distribution was maintained during the deposition of Pd NPs (see pore size distribution curve, inset in Fig. 2).
The BET surface area, average pore size and pore volume of pristine ZrSBA-15 are 617 m2 g−1, 8.5 nm and 1.10 cm3 g−1, respectively. Upon Pd introduction to ZrSBA-15, a gradual decrease in surface area, pore size and pore volume is observed, which suggests that the Pd NPs are dispersed and introduced into the pore channels of ZrSBA-15 and also that the metal was loaded successfully without disintegrating the structure. This is in agreement with the XRD analysis results. Compared to 3.3 wt% Pd/ZrSBA-15, the surface area, pore size and pore volume of 3.3 wt% Pd/ZrSBA-15/5t slightly shifted to larger values (see Table S1†). Possibly, tiny amounts of Pd NPs leached away from the support in the process of five cycle reactions, whereas the channel structure could also provide internal surface area and active sites (see EDX results shown in Fig. S1†).
Additionally, the SEM results confirmed the platelet-like morphology of ZrSBA-15, 3.3 wt% Pd/ZrSBA-15 and 3.3 wt% Pd/ZrSBA-15/5t (see Fig. 3), clearly suggesting that the macroscopic features have been preserved during the loading of active Pd species. Correspondingly, the TEM images of the catalyst composite revealed well-ordered mesoscale pores arranged in a hexagonal pattern and aligned along the thickness of the platelet. Especially, as demonstrated by the image in Fig. 3e, the Pd NPs are evenly dispersed on the surface and channels of ZrSBA-15 support, no obvious aggregation is observed throughout the image. Favorably, the well-dispersed Pd NPs on the platelet-like support can enable reactant molecules to combine with active sites more easily and enable product molecules to desorb from the active sites more quickly, possibly leading to enhanced reaction rate and long-term durability during subsequent testing conditions. The significantly shortened pore length could have facilitated the heterogeneous catalysis, owing to the reduction of mass transfer and diffusion resistance.
 | ||
| Fig. 3 SEM images of (a) ZrSBA-15, (b) 3.3 wt% Pd/ZrSBA-15 and (c) 3.3 wt% Pd/ZrSBA-15/5t; TEM images of (d) ZrSBA-15, (e) 3.3 wt% Pd/ZrSBA-15 and (f) 3.3 wt% Pd/ZrSBA-15/5t. | ||
To determine the loading amount of Pd species, Fig. S1† shows the EDX results of 3.3 wt% Pd/ZrSBA-15 and 3.3 wt% Pd/ZrSBA-15/5t samples. The composition of this sample was confirmed by the EDX spectrum, which revealed that Pd, Zr, O, C and Si were predominant species. The Pd content on the surface determined by EDX had an average value of 2.47 wt% (Fig. S1a†); this means that some of the Pd NPs were attached on the inner wall of the channels of ZrSBA-15, as well as another part of the loss in the preparation of catalysts. After five cycle reactions, the amount of Pd slightly decreased; this was consistent with the BET data listed in Table S1.† The loading amount (2.91 wt%) of 3.3 wt% Pd/ZrSBA-15 was further determined by ICP-OES analysis, and the ICP-OES result for 3.3 wt% Pd/ZrSBA-15/5t was 2.89 wt% (Table 1).
| Sample | Pd loadingb (%) | Conversion% | Selectivity% | TONa |
|---|---|---|---|---|
| a TON (turnover number) = moles of benzaldehyde produced per mole of palladium after 4 h reaction.b Determined by ICP.c Reaction conditions: time 4 h; catalyst 0.3 g; n (benzyl alcohol) = 0.04 mol. | ||||
| Without catalyst | — | 0 | — | — |
| ZrSBA-15 | — | Trace | — | — |
| 3.3 wt% Pd/SBA-15 | — | 86.3% | 98% | 41 |
| 3.3 wt% Pd/ZrSBA-15 | 2.91 | 95.2% | >99% | 54 |
| 3.3 wt% Pd/ZrSBA-15/5t | 2.89 | 80.9% | >99% | 39 |
FT-IR was used to detect the surface chemistry of the materials. As shown in Fig. S2,† the Si–O–Si (1087, 803, 475 cm−1) and Si–O–Zr (1083 cm−1) bands, typical of pristine ZrSBA-15, are clearly identified. All the samples display a absorption bank at 3408, 1627 cm−1, belonging to the silanol groups and physisorbed water in the hydroxy region, are also identified. Further, new bands at 1715 and 1396 cm−1 were also observed for the Pd/ZrSBA-15, possibly indicating that Pd species had been successfully anchored over the surface of the ZrSBA-15.
The electronic state of the Pd species on ZrSBA-15 was measured using the XPS technique. Firstly, the XPS survey spectrum clearly identified the presence of Zr, Si, Pd, O, and C elements in the sample (Fig. 4a), no other impurities were observed.42 The Zr 3d spectrum of 3.3 wt% Pd/ZrSBA-15, along with the applied de-convolution peaks, is shown in Fig. 4b. The distinct peaks at 182.5 and 185.3 eV corresponded to Zr 3d5/2 and Zr 3d3/2, respectively. These can be attributed to the interactions between the zirconium ions and the speciation of zirconium into the framework of SBA-15, respectively. The Pd pattern of the 3.3 wt% Pd/ZrSBA-15 is displayed in Fig. 4c. The peaks at 336.9 eV and 342.1 eV can be attributed to the Pd 3d5/2 and Pd 3d3/2, revealing the presence of Pd0 in the sample. Possibly, the shoulder at 342.1 eV (Pd 3d3/2) was due to the surface oxidation of the palladium nanoparticles upon exposure to air. Binding energy values of Pd 3d5/2 and Pd 3d3/2 due to metallic Pd suggested that the prepared 3.3 wt% Pd/ZrSBA-15 might exhibit favorable catalytic activity in subsequent C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond formation.
O bond formation.
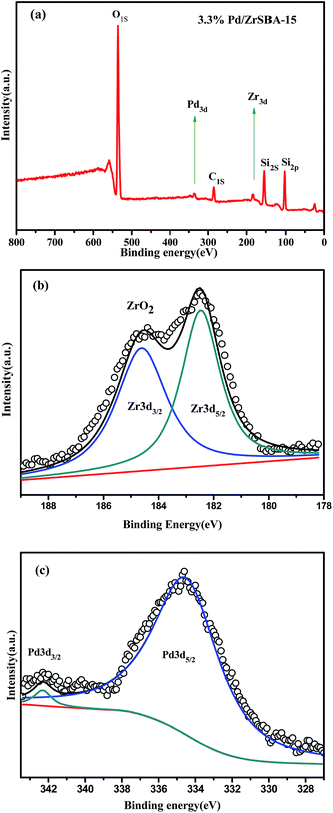 | ||
| Fig. 4 XPS spectra of 3.3 wt% Pd/ZrSBA-15: (a) full spectrum and the spectral regions corresponding to (b) Zr 3d and (c) Pd 3d. | ||
3.2 Catalytic performance for benzyl alcohol aerobic oxidation
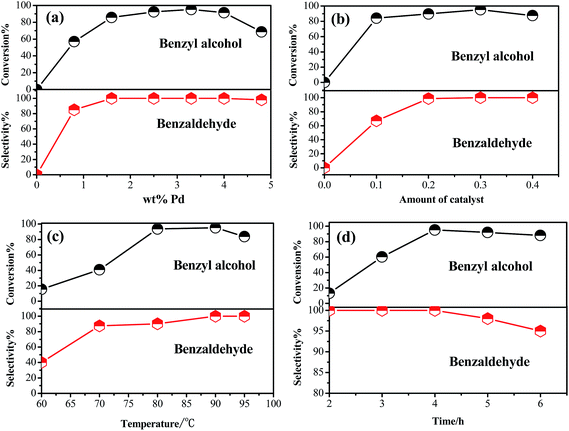
The catalytic aerobic oxidation of BnOH over the resultant Pd/ZrSBA-15 was investigated at 60 °C using a glass reactor under solvent- and base-free conditions. From the results presented in Fig. 5a, it was evident that the 3.3 wt% Pd/ZrSBA-15 catalyst showed superior catalytic activity with 95% conversion and approached 100% selectivity. As a consequence, a loading of 3.3 wt% Pd onto ZrSBA-15 was used in all subsequent reactions. Subsequently, the influence of the amount of the 3.3 wt% Pd/ZrSBA-15 catalyst added to the reaction was investigated. Excellent conversion and selectivity were observed when 0.3 g of the catalyst was added (Fig. 5b). Next, the effect of temperature on the catalytic oxidation of BnOH was monitored (Fig. 5c). Clearly, a substantially improved conversion of BnOH occurred with an increase in temperature. For all temperatures below 60 °C, the yield of BzH increased as the temperature was increased. In the 75–90 °C range, a significant increase in the oxidation activity was observed, resulting in the increased formation of the main product BzH; at this stage, the selectivity to BzH reaches almost 100%. All subsequent investigations into the oxidation of BnOH with 3.3 wt% Pd/ZrSBA-15 were performed at 90 °C.Besides this, the significance of the reaction time on the selective oxidation of BnOH was studied (Fig. 5d). Considering that higher reaction efficiency should have been achieved at shorter durations of the reaction, we chose 4 h as the suitable reaction time for all subsequent reactions. For reactions that were run for periods beyond 4 h, there was no improvement in conversion or selectivity. Favorably, the by-product benzoic acid was not detected at any point during the required reaction time under the testing temperatures. This suggested that Pd/ZrSBA-15 with shortened pore channels could suppress the over-oxidation of BnOH. However, the origin of this phenomenon is still under investigation.
In order to further evaluate the performance of the catalyst, recovery and reuse of the catalyst were explored. If the active sites were not properly anchored onto the support, they would have leached away during the reaction process, resulting in loss of activity in subsequent runs. Therefore, the catalyst was recovered by filtration after each run, and then it was reused in the next run under the same ratio of catalyst to BnOH and other conditions. Only a minor decrease (ca. 0.4%) in the activity for BzH (>99% initially) was observed upon recycling the catalyst in the fifth run (Fig. 6a). By contrast, the conversion rates of Pd/SBA-15, which was prepared by loading active Pd particles onto siliceous SBA-15 decreased instantly, whereas the selectivity lowered more slightly under testing conditions (Fig. 6b). This revealed that there was a strengthened metal–support interaction between Pd particles and ZrSBA-15 within Pd/ZrSBA-15, favorably preventing the leaching of Pd NPs during the reaction process. In contrast, no favorable interaction existed in the Pd/SBA-15 counterpart, leading to a significant loss of large number of active sites from the support over the five cycles of the reaction.
 | ||
| Fig. 6 Recyclability of 3.3 wt% Pd/ZrSBA-15 (a) and 3.3 wt% Pd/SBA-15 (b) for the selective oxidation of BnOH under solvent-free reaction conditions. | ||
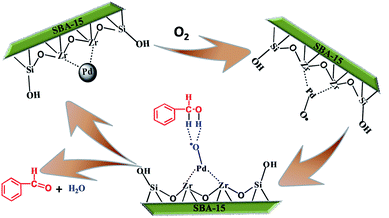
Based on the investigation into the various parameters of the aerobic oxidation process and relevant literature reports,43 a reasonable mechanism for the selective oxidation of BnOH to BzH was proposed (see Scheme 2). Firstly, the surface of the catalytic materials could activate oxygen and also regenerate to the original state during the course of the reactions. The calculated small dissociation barrier of O2 on Pd (110) facets indicates that oxygen can be easily dissociated on Pd (110). Secondly, the Pd–O bond is formed, which is demonstrated in Fig. 4. The two hydrogen atoms of the O–H bond and α-C–H bond in benzyl alcohol combine with one negatively charged oxygen atom. When Pd0 was oxidized to Pd2+, the Pd2+-based catalyst would be a good catalyst in the dehydrogenation reaction.44 Finally, the reaction of the species with the activated oxygen regenerates the Pd0 species, along with the formation of H2O. During the reaction process, BnOH can be adsorbed onto the highly distributed and ultra-small Pd NPs, desirably leading to the transformation to BzH.
In the end, under optimal reaction conditions, the activity of different samples, along with the control of catalyst-free conditions, were conducted to further identify the superior catalytic performance of the synthesized Pd/ZrSBA-15. As illustrated by the results in Table 1, it can be concluded that the highest efficiency was observed for 3.3 wt% Pd/ZrSBA-15 (see entry 4). Additionally, no detectable data for conversion and selectivity could be gained in the absence of active Pd NPs (see entry 2). It is noteworthy that the TON results in Table 1 also offer favorable evidence that the Pd/ZrSBA-15 has higher activity than Pd/SBA-15 in the production of BzH. Overall, within the flake structure, the Pd NPs can lead to a more uniform distribution, through which more active sites are exposed, and better catalytic performance is achieved.
4. Conclusions
In summary, we have successfully synthesized Pd/ZrSBA-15 with different Pd content by a simple adsorption-deposition method, utilizing the favorable interaction between Pd NPs and the acidic framework ZrSBA-15. The characterization results showed that ultra-small Pd NPs with an average size of 1.9–2.3 nm had been desirably loaded inside the pore channels of platelet-like ZrSBA-15 with a low Pd amount (3.3 wt%). This composite was used for the aerobic oxidation of BnOH, and excellent conversion of BnOH and selectivity for BzH was gained under testing conditions, without significant loss in activity even after five reaction cycles. It demonstrated the efficiency and recyclability of the 3.3 wt% Pd/ZrSBA-15 composite. Within this context, it was postulated that the presence of Zr atoms in ZrSBA-15 led to an efficient dispersion of Pd particles by enhancing metal–support interaction; consequently, the process of preparation is simple and a marked effect on the catalytic performance was observed. By virtue of its short meso-channels, easy separation, and reusability, the composite catalyst may show promising performance in the aerobic oxidation of related alcohol compounds.Acknowledgements
Financial support from the National Natural Science Foundation of China (21446001), the Program for Liaoning Innovative Research Team in University (LT2013012) and the Program for Liaoning Excellent Talents in University (LJQ2014056) is highly appreciated.References
- I. Lee, F. Delbecq, R. Morales, M. A. Albiter and F. Zaera, Nat. Mater., 2009, 8, 132–138 CrossRef CAS PubMed.
- A. Solhy, B. F. Machado, J. Beausoleil, Y. Kihn, F. Goncalves, M. F. R. Pereira, J. J. M. órfão, J. L. Figueiredo, J. L. Faria and P. Serp, Carbon, 2008, 46, 1194–1207 CrossRef CAS.
- F. A. Luzzio, R. W. Fitch, W. J. Moore and K. J. Mudd, J. Chem. Educ., 1999, 76, 974 CrossRef CAS.
- C. Della Pina, E. Falletta and M. Rossi, J. Catal., 2008, 260, 384–386 CrossRef CAS.
- S. E. Davis, M. S. Ide and R. J. Davis, Green Chem., 2013, 15, 17–45 RSC.
- A. Abad, P. Concepcion, A. Corma and H. Garcia, Angew. Chem., Int. Ed., 2005, 44, 4066–4069 CrossRef CAS PubMed.
- N. Zheng and G. D. Stucky, Chem. Commun., 2007, 3862–3864 RSC.
- (a) M. Boronat, A. Corma, F. Illas, J. Radilla, T. Rodenas and M. J. Sabater, J. Catal., 2011, 278, 50–58 CrossRef CAS; (b) V. R. Choudhary and D. K. Dumbre, Catal. Commun., 2011, 13, 82–86 CrossRef CAS.
- H. Wang, W. Fan, Y. He, J. Wang, J. N. Kondo and T. Tatsumi, J. Catal., 2013, 299, 10–19 CrossRef CAS.
- T. Nishimura, N. Kakiuchi, M. Inoue and S. Uemura, Chem. Commun., 2000, 1245–1246 RSC.
- (a) K. Mori, K. Yamaguchi, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2002, 124, 11572–11573 CrossRef CAS PubMed; (b) K. Mori, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2004, 126, 10657–10666 CrossRef CAS PubMed.
- (a) H. Wu, Q. Zhang and Y. Wang, Adv. Synth. Catal., 2005, 347, 1356–1360 CrossRef CAS; (b) S. F. J. Hackett, R. M. Brydson, M. H. Gass, I. Harvey, A. D. Newman, K. Wilson and A. F. Lee, Angew. Chem., Int. Ed., 2007, 46, 8593–8596 CrossRef CAS PubMed.
- (a) B. I. Enache, J. K. Edwards, P. Landon, B. Solsona-Esprriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight and G. J. Hutchings, Science, 2006, 311, 362–365 CrossRef PubMed; (b) P. J. Miedziak, Q. Heb, J. K. Edwardsa, S. H. Taylora, D. W. Knighta, B. Tarbitc, C. J. Kielyb and G. J. Hutchings, Catal. Today, 2011, 163, 47–54 CrossRef CAS.
- Y. X. Li, Y. Gao and C. Yang, Chem. Commun., 2015, 51, 7721–7724 RSC.
- C. C. de Paula, A. Garcia Ramos, A. C. da Silva, E. Cocchieri Botelho and M. C. Rezende, Carbon, 2002, 40, 787–788 CrossRef CAS.
- B. Yoon and C. M. Wai, J. Am. Chem. Soc., 2005, 127, 17174–17175 CrossRef CAS PubMed.
- J. P. Tessonnier, L. Pesant, G. Ehret, M. J. Ledoux and C. Pham-Huu, Appl. Catal., A, 2005, 288, 203–210 CrossRef CAS.
- A. Tavasoli, R. M. M. Abbaslou, M. Trepanier and A. K. Dalai, Appl. Catal., A, 2008, 345, 134–142 CrossRef CAS.
- D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852–7872 CrossRef CAS PubMed.
- R. J. White, R. Luque, V. L. Budarin, J. H. Clark and D. J. Macquarrie, Chem. Soc. Rev., 2009, 38, 481–494 RSC.
- Z. P. Sun, X. G. Zhang, H. Tong, R. L. Xue, Y. Y. Liang and H. L. Li, Appl. Surf. Sci., 2009, 256, 33–38 CrossRef CAS.
- V. Mazumder and S. Sun, J. Am. Chem. Soc., 2009, 131, 4588–4589 CrossRef CAS PubMed.
- Z. P. Sun, X. G. Zhang, H. Tong, R. L. Xue, Y. Y. Liang and H. L. Li, Appl. Surf. Sci., 2009, 256, 33–38 CrossRef CAS.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548–552 CrossRef CAS PubMed.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024–6036 CrossRef CAS.
- P. Han, X. Wang, X. Qui, X. Ji and L. Gao, J. Mol. Catal. A: Chem., 2007, 272, 136–141 CrossRef CAS.
- P. Wang, Z. Dong, Y. Lei, Y. Du, H. Li, H. Yang, Y. Nie and J. Ma, J. Porous Mater., 2013, 20, 277–284 CrossRef CAS.
- V. Polshettiwar, D. Cha, X. Zhang and J. M. Basset, Angew. Chem., Int. Ed., 2010, 49, 9652–9656 CrossRef CAS PubMed.
- R. Palcheva, A. Spojakina, L. Dimitrov and K. Jiratova, Microporous Mesoporous Mater., 2009, 122, 128–134 CrossRef CAS.
- F. Su, Q. T. Wu, D. Song, X. H. Zhang, M. Wang and Y. H. Guo, J. Mater. Chem. A, 2013, 1, 13209–13221 CAS.
- A. Sinhamahapatra, P. Pal, A. Tarafdar, H. C. Bajaj and A. B. Panda, ChemCatChem, 2013, 5, 331–338 CrossRef CAS.
- J. Dhainaut, J. P. Dacquin, A. F. Lee and K. Wilson, Green Chem., 2010, 12, 296–303 RSC.
- S. W. Bian, Z. Ma, L. S. Zhang, F. Niu and W. G. Song, Chem. Commun., 2009, 1261–1263 RSC.
- K. Lin, P. P. Pescarmona, K. Houthoofd, D. Liang, G. Van Tendeloo and P. A. Jacobs, J. Catal., 2009, 263, 75–82 CrossRef CAS.
- G. De Cremer, M. B. J. Roeffaers, E. Bartholomeeusen, K. Lin, P. Dedecker, P. P. Pescarmona, P. A. Jacobs, D. E. De Vos, J. Hofkens and B. F. Sels, Angew. Chem., Int. Ed., 2010, 49, 908–911 CrossRef CAS PubMed.
- C. Y. Ma, Z. Mu, J. J. Li, Y. G. Jin, J. Cheng, G. Q. Lu, Z. P. Hao and S. Z. Qiao, J. Am. Chem. Soc., 2010, 132, 2608–2613 CrossRef CAS PubMed.
- L. Y. Meng, S. R. Zhai, S. Li, B. Zhai, Q. D. An and X. W. Song, Eur. J. Inorg. Chem., 2014, 71, 354–363 CAS.
- D. Zhao, Q. Huo and J. Feng, J. Am. Chem. Soc., 1998, 120, 6024–6036 CrossRef CAS.
- H. Wang and C. J. Liu, Appl. Catal., B, 2011, 106, 672–680 CrossRef CAS.
- K. Murata, I. Takahara and M. Inaba, React. Kinet. Catal. Lett., 2008, 93, 59–66 CrossRef CAS.
- R. B. Cang, B. Lu and X. P. Li, Chem. Eng. Sci., 2015, 137, 268–275 CrossRef CAS.
- J. Zhu, J. L. Faria, J. L. Figueiredo and A. Thomas, Chem.–Eur. J., 2011, 17, 7112–7117 CrossRef CAS PubMed.
- A. Abad, A. Corma and H. Garcìa, Chem.–Eur. J., 2008, 214, 12–222 Search PubMed.
- F. F. Wang, Z. S. Lu, L. Yang, Q. H. Tang and Y. Guo, Chem. Commun., 2013, 49, 6626–6628 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra17272e |
| This journal is © The Royal Society of Chemistry 2016 |