Self-assembly of multifunctional integrated nanoparticles loaded with a methotrexate–phospholipid complex: combining simplicity and efficacy in both targeting and anticancer effects†
Yanxiu Li‡
a,
Jinyan Lin‡a,
Guihua Liub,
Yang Lia,
Liang Songa,
Zhongxiong Fanac,
Xuan Zhub,
Guanghao Su*d and
Zhenqing Hou*ac
aDepartment of Biomaterials, College of Materials, Xiamen University, Xiamen 361005, China. E-mail: houzhenqing@xmu.edu.cn
bDepartment of Pharmacy, School of Pharmaceutical Science, Xiamen University, Xiamen 361005, China
cDepartment of Physics, Changji University, Changji 831100, China
dInstitute of Pediatric Research, Children's Hospital of Soochow University, 92 Zhongnan Street, Suzhou 215025, China
First published on 6th September 2016
Abstract
Recently, the global trend in the field of nanomedicine has been toward the design of highly sophisticated drug delivery systems with active targeting and therapeutic functions, as well as responsiveness to biological stimuli for improving therapeutic efficacy. But offering sophistication generally increases their complexity that might be disadvantageous in pharmaceutical development. In this paper, we hypothesize that using a clinical anticancer drug methotrexate (MTX) as both a targeting ligand and a therapeutic agent to interact with natural product phospholipid (PC) and thus self-assemble will lead to an efficient but simple and flexible, moreover, multifunctional integrated system. The methotrexate (MTX)–phospholipid (PC) complex is prepared by a co-solvent method and can be self-assembled into nanoparticles (MTX–PC NPs), which significantly increases the drug-loading ability and reduces the burst drug release compared with free MTX and MTX-loaded liposomes. The MTX–PC complex and its self-assembled MTX–PC NPs were evaluated by UV, TGA, DSC, XRD, FTIR, 1H-NMR, SEM, TEM, AFM, and in an in vitro drug release study. The MTX-PC NPs had a particle size of 152.5 ± 3.2 nm, a narrow size distribution, a high drug-loading efficiency of 20.7 ± 2.4%, and a controlled and sustained release behavior. The in vitro cellular uptake results showed that a vital advantage of this system is that MTX-PC NPs with pH-triggered drug release can exert an early-phase good active targeting efficiency (attributed to the surface-absorbed MTX) cooperating with a late-phase excellent anticancer efficiency (was attributed to the core-dispersed MTX). The concept of the self-targeted anticancer effect based on drug–lipid hybrid systems might be a promising candidate for cancer therapy compared with traditional drug delivery systems.
1. Introduction
Cancer continues to be a prevalent and lethal disease worldwide. Complete surgical resection would still be one of the best options to treat cancer for long-term survival, with a 5 year survival rate of 20–40%.1 Unfortunately, only a small percentage of patients suffering from cancer are suitable for surgery at the proper time, even so, they also encounter a high rate of tumor recurrence.2 In addition, radiotherapy and chemotherapy would still be widely used to treat the residual, infiltrative tumor cells after surgical resection.3,4 But the chemotherapeutic efficacy is largely limited by nonspecific cellular uptake and tissue biodistribution, rapid metabolization and excretion from the body, serious side effects, poor tumor selectivity, and low tumor cell uptake efficiency of anticancer drugs.5,6 Therefore, the development of novel cancer chemotherapy and drug delivery is urgently needed.7,8In recent decades, methotrexate (MTX) has attracted considerable research interest because of its potent anticancer and anti-rheumatic/anti-inflammatory effect.9,10 Particularly, as an antimetabolite and antifolate anticancer drug, MTX could be delivered to the cytoplasm and interact with dihydrofolate reductase (DHFR) to inhibit the metabolism of folic acid (FA), leading to DNA synthesis inhibition and cell death.11,12 In addition, MTX is clinically used against a wide range of cancer including breast cancer, cervical cancer, head and neck cancer, liver cancer, etc.5,10,13 However, limitations in relatively low water solubility (0.01 mg mL−1), low permeability (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 0.5), short plasma half-life (2–10 h), and nonspecific biodistribution of MTX related with systemic toxicity issues are some obstacles encountered with clinical use that need to be overcome.14,15
P = 0.5), short plasma half-life (2–10 h), and nonspecific biodistribution of MTX related with systemic toxicity issues are some obstacles encountered with clinical use that need to be overcome.14,15
It is well-known that phospholipid is a vital component of cell membrane with good biocompatibility/biodegradability and low toxicity. As an amphipathic molecule, PC possessed a zwitterionic charged headgroup and two neutrally charged tail groups, a rare molecular characteristic that renders PC miscible in both water and lipid environments, in which the oxygen atom of the phosphate group has a strong tendency to gain electrons and the nitrogen atom to lose electrons. Additionally, any kind of drug possessing an active hydrogen atom (–COOH, –OH, –NH2, –NH–, etc.) can be interacted to the phosphate or quaternary ammonium group of a PC molecule, generating an amphiphilic agent to facilitate the crossing of the cell-membrane barrier.16,17 At present, PC has been clinically available for several decades, owing to these outstanding advantages including biocompatibility, biodegradability, metabolic activity, and low toxicity and cytotoxicity compared to its synthetic alternatives. These abundant characteristics make PC an attractive candidate for production of pharmaceutical dosage forms. So drug–phospholipid complex was an advantageous option to improve both the solubility and permeability of therapeutic drug. Thus, drug–phospholipid complex has received significant attention due to the superior amphiphilic characteristic of phospholipid and the simplicity, flexibility, effectiveness of manufacturing process.16,17 A variety of therapeutic drug-phospholipid complexes (e.g. others reported antidiabetic drug insulin–phospholipid complex,18 hepatoprotective drug berberine–phospholipid complex,19 and our reported anticancer drug mitomycin C–phospholipid complex,20 etc.) have been previously investigated, in which the drug molecules are entrapped within the phospholipid molecules. In the previous study, we first reported chemotherapeutic drug (mitomycin C)–phospholipid complex self-assembled phytosomal nanoparticles.20 Compared to the free drug, these phytosomal nanoparticles improved drug stability, increased drug-loading ability and enhanced drug delivery efficacy. In addition, a phytosome unit is similar to a liposome in general, but with a different guest localization; in a liposome, the ingredients are hosted in the inner cavity, with limited possibility of molecular interaction between the surrounding lipid and a hydrophilic guest. In a phytosome, the ingredients are dispersed into phospholipid (a dietary surfactant and can be compared to an integral part of the lipid membrane), where the polar groups of the hydrophilic guest interact with the phosphate heads of phospholipid via electrostatic and hydrogen-bonding interactions. Therefore, phytosomal nanoparticle, a type of drug–phospholipid complex-based drug delivery system possesses lower lipid material dosage and higher drug loading ability compared to a liposomal nanoparticle, offering an effective platform for increasing the therapeutic drug effectiveness.16,21
To decrease the systemic side effects against non-target tissues and cells as well as increasing the chemotherapeutic effect, targeting drug delivery system which can help providing a sufficient drug concentration accumulated at the tumor site and internalized in the tumor cell is highly desired.22,23 These targeted strategies were mostly responsible for the enhanced permeability and retention effect (e.g. liposomes, polymeric nanoparticles, micelles, etc.), surface-functionalized receptor-specific ligand (e.g. RGD peptide, galactose, and hyaluronic acid, folate, transferrin, etc.), surface-functionalized microenvironment stimulus (e.g. pH, redox, temperature, enzyme, etc.)-responsive moieties, or surface-functionalized cell-penetrating peptides.24–27 On the basis of our previous experimental works,28 we were greatly motivated and interested in MTX that could target as an early-phase selective targeting ligand as well as function as a late-phase therapeutic anticancer drug. It was reported that MTX and FA could enter cells via the same transport pathways (reduced FA carrier, proton-coupled FA transporter, and membrane-associated FA receptor) as a result of their high structural similarity.29–31 The dual function drug delivery systems would be a promising candidate for cancer therapy by simplifying targeted drug delivery cooperated with cancer chemotherapy.32
In our present work, we reported the synthesis of MTX–PC complex via a co-solvent method and the further construction of MTX–PC complex-loaded nanoparticles via a self-assembly technique for enhancing cellular uptake and increasing anticancer activity (Fig. 1). To the best of our knowledge, this work for the first time reported the anticancer drug–phospholipid complex-based nanoscaled drug delivery system for exerting the self-targeting (was attributed to surface-absorbed MTX) and therapeutic (was attributed to core-dispersed MTX) effect (Fig. 2).
 | ||
| Fig. 1 (A) Illustration of the preparation of the MTX–PC complex and its self-assembled nanoparticles (MTX–PC NPs). (B) Chemical structures for MTX and PC (consisting of 90–95% phosphatidylcholine). | ||
2. Materials and methods
2.1. Materials
All chemical reagents were of analytical grade without further purification unless stated. Methotrexate (MTX) and folate (FA) were purchased from Bio Basic Inc. (Markham, Ontario, Canada). Soybean phosphatidylcholine (PC) consisting of 90–95% phosphatidylcholine was obtained by Lipoide GmsH (Ludwigshafen, Germany). DiD was from Molecular Probes Inc. (Eugene, OR, USA). Dichloromethanea (DCM) and tetrahydrofuran (THF) were obtained from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). RPMI-1640, penicillin-streptomycin, and tryposin were ordered from Sigma Chemical Corp (St. Louis, MO, USA).2.2. Cell cultures
Human cervical carcinoma cell line HeLa cells and human breast carcinoma cell line MCF-7 cells were obtained from American Type Culture Collection (ATCC). HeLa cells were grown in FA-deficient Dulbecco's Modified Eagle Media (DMEM without FA) supplemented with 10% FBS and 1% penicillin/streptomycin. The cells were cultivated in an incubator (Thermo Scientific) at 37 °C in the presence of 5% CO2 for 24 h. MCF-7 cells were cultured in FA-deficient Roswell Park Memorial Institute 1640 medium (RPMI 1640 without FA) under similar conditions.2.3. Synthesis of MTX–PC complex
The MTX–PC was synthesized by a co-solvent technique based on our previous reported study with some modifications.20 Firstly, 10 mg of MTX and 30 mg of PC were added into 12 mL of tetrahydrofuran (THF) and continuously stirred. The resulting mixture became clear and transparent in 24 h, indicating that the MTX–PC complex dispersion was formed. Subsequently, the organic solvent was removed through vacuum rotary evaporation, obtaining the film of the crude MTX–PC complex. The physical mixture of MTX and PC was prepared at the same weight ratio by grinding them inside an agate mortar.In order to determine the complexation rate of MTX and PC in the formation of the MTX–PC complex, the crude MTX–PC complex was added into dichloromethane (DCM, insoluble solvent of MTX), followed by the vigorous vortexing, and filtered through a 220 nm pore-size membrane filter to remove the excess and uncomplexed MTX. The filtrate was evaporated to dryness according to HPLC analysis. The purified MTX–PC complex was determined by our reported analytical method.33 The compexation rate (CR) of the MTX–PC complex was calculated by the following equation:
| CR (%) = W(complexed)/W(total) × 100% |
2.4. Physicochemical characterization of MTX–PC complex
The UV-vis absorption spectrum was recorded with a Perkin Elmer Lambda 750 UV-vis-near-infrared spectrophotometer (Perkin-Elmer, Norwalk CT). The differential scanning calorimetry (DSC) curves and thermal gravity analysis (TGA) curves were DSC analysis (DSC 204F1, Netzsch, Selb, Germany). The X-ray diffraction (XRD) spectra were recorded by an X-ray diffractometer (Phillips X′ pert Pro Super, Panalytical, Almelo, Netherlands). The fourier transform infrared spectrum was performed on a Bruker IFS-55 infrared spectrometer (Bruker, Zurich, Switzerland). The 1H NMR spectrum was determined on a Bruker AV400 MHz NMR spectrometer (Bruker, Billerica, MA, USA). The solvent for MTX and physical mixture of MTX and PC was deuterated dimethylsulfoxide (DMSO), and that for PC and MTX–PC complex was deuterated chloroform (CHCl3). The morphology was observed by transmission electron microscopy (TEM, JEM 1400, JEOL, Tokyo, Japan). The fluorescence dye-labeled MTX–PC complex was observed using a Leica TCS SP5 confocal laser scanning microscopy (Leica Microsystems, Mannheim, Germany). The free MTX, PC, and physical mixture of MTX and PC were used as controls for comparison.2.5. Self-assembly of amphiphilic MTX–PC complex
The MTX–PC NPs were constructed by the amphiphilic MTX–PC complex a novel lipid–drug complex film dispersion method. Briefly, the MTX–PC complex (equivalent to 10 mg of MTX) was added into 10 mL of DCM. The organic solvent was removed by vacuum rotary evaporation to form a dry lipid film. The dried film was dispersed with DI water in an ultrasonic bath cleaner at ultrasonic power (200 W) at 40 °C for 20 min. After that, the resulting MTX–PC NPs extruded through by extrusion through 220 nm pore-size membrane filters. The MTX–PC NPs suspension were washed three times by ultrafiltration using an Amicon Ultra-4 centrifugal filter (Millipore, Billerica, MA) with a molecular weight cut-off of 10 kDa to remove residual unencapsulated molecules. For in vitro cytotoxicity, the MTX–PC NPs without MTX was prepared using the same procedures as above except that the MTX–PC complex was replaced by the free MTX. For in vitro intracellular delivery, DiD, a hydrophobic and lipophilic fluorescent probe, was loaded within the MTX–PC NPs. The MTX–PC NPs@DiD was prepared using the procedures described above.2.6. Drug-loading content
The amount of the drug was determined by HPLC analysis our according to our reported analytical method.33 Drug-loading content of the MTX–PC NPs were calculated using eqn (1).
 | (1) |
2.7. Physicochemical characterization of self-assembled MTX–PC NPs
The hydrodynamic particle size and polydispersity index (PDI) was assayed by dynamic light scattering (DLS) using a Malvern Zetasizer Nano-ZS (Malvern Instruments, Worcestershire, U.K.). The zeta potential was determined by electrophoretic light scattering (ELS) using a same equipment. The morphology was visualized using scanning electron microscopy (SEM, LEO 1530VP, Oberkochen, Germany) and transmission electron microscopy (TEM, JEM 1400, JEOL, Tokyo, Japan). The X-ray diffraction (XRD) spectra were recorded by an X-ray diffractometer (Phillips X′ pert Pro Super, Panalytical, Almelo, Netherlands).2.8. In vitro stability tests
The in vitro stability of the MTX–PC NPs was performed under different media by incubating the MTX–PC NPs in water and phosphate-buffered saline (PBS) solution for 48 h. The hydrodynamic particle size of the MTX–PC NPs was determined at predesigned time intervals by DLS analysis.2.9. In vitro release profiles
The release profile of MTX from the MTX–PC NPs was determined by a dialysis technique using a dialysis membrane (molecular weight cut-off of 3000 Da). The experiment was evaluated in PBS solution (pH 7.4, 6.5, and 5.0). 1 mL of the MTX–PC NPs was transferred into a dialysis membrane and then immersed into 49 mL of PBS at 37 °C with gentle shaking. At the predesigned time intervals, 1 mL of the release medium was withdrawn and subsequently the release medium was replaced with 1 mL of fresh medium. The concentration of released MTX was determined by a HPLC method as described above. The accumulative drug release of the MTX–PC NPs was expressed as a percentage of the released drug. The drug release profiles of the free MTX and MTX–PC liposomes were used as controls for comparison. The cumulative release curve was calculated using eqn (2).
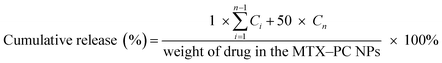 | (2) |
2.10. In vitro cellular uptake
DiD as a hydrophobic and lipophilic fluorescence dye was encapsulated within the MTX–PC NPs. Human cervical cancer cell lines HeLa cells or human breast cancer cell lines MCF-7 cells were cultured in 6-well plates with at a density of 1 × 105 cells per well. The cells were incubated at 37 °C and 5% CO2 for 24 h. The MTX–PC NPs@DiD suspensions were added to the cells for predesigned incubation time periods. After incubation, the cells were washed with PBS, fixed with 4% paraformaldehyde, stained with DAPI and imaged using a Leica TCS SP5 confocal laser scanning microscopy (Leica Microsystems, Mannheim, Germany). The cells incubated with the MTX–PC NPs@DiD in the presence of the free FA at the equivalent DiD concentration were used as controls for comparison.2.11. Flow cytometry analysis
HeLa cells were seeded in 6-well plates with a density of 1 × 105 cells per mL and incubated for 24 h, and then treated with the MTX–PC NPs@DiD for predesigned incubation time periods. After incubation, HeLa cells were harvested with trypsin-EDTA and washed with PBS. Then the fluorescence intensity was measured using a Beckman Coulter Cell Lab Quanta SC with excitation wavelengths of 633 nm. HeLa cells incubated with the MTX–PC NPs@DiD in the presence of the free FA at the equivalent DiD concentration were used as controls for comparison.2.12. Intracellular drug delivery
To confirm the cellular uptake and subsequent intracellular drug release, fluorescein isothiocyanate (FITC) labeled MTX (FITC–MTX was coupled between MTX and FITC via a thiourea linkage)33–35 was synthesized for use as a fluorescent probe. In addition, DiD was also encapsulated within the FITC–MTX–PC NPs. HeLa cells were seeded, preincubated at 37 °C and 5% CO2 for 8 h, and then incubated the FITC–MTX–PC NPs@DiD for 16 h. The cells were washed with PBS, fixed with 4% paraformaldehyde, stained with DAPI, and imaged using a Leica TCS SP5 confocal laser scanning microscopy.2.13. In vitro cell cytotoxicity assay
The in vitro cytotoxicity of the MTX–PC NPs was measured using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay according to the manufacturer's suggested procedures. HeLa cells or MCF-7 cells were incubated with the MTX–PC NPs at different MTX concentrations (1.25, 2.5, 5, 10, 20 μg mL−1) for 24 h at 37 °C and 5% CO2. The data was expressed as the percentage of surviving cells. The cells incubated with the free MTX and MTX–PC NPs without MTX (PC NPs) at the equivalent MTX concentration were used as controls for comparison.2.14. Statistical analysis
All experiments were repeated at least three times. All data were expressed as mean ± s.d. Statistical tests were performed by the Student's t-test. Data are presented as the mean ± SD. The statistical difference would be considered statistically significant when the P value was than 0.05.3. Results and discussion
3.1. Synthesis of MTX–PC complex and preparation of MTX–PC complex self-assembled nanoparticles
The synthetic route of MTX–PC complex and MTX–PC complex self-assembled nanoparticles was illustrated in Fig. 1A. MTX–PC complex was first synthesized by a co-solvent method using MTX and phospholipid. Subsequently, the self-assembled nanoparticles were constructed by the self-assembly of MTX–PC complex via a novel lipid–drug complex film dispersion method. It was expected that the anticancer drug–phospholipid complex with the self-assembly could not only increase the lipophilicity and hydrophobicity (improve the amphiphilicity) of MTX drug for enhancing drug loading efficiency but also increase the permeability of MTX for increasing drug delivery efficacy.In addition, to visually observe the effect of reaction time on the complexation effect between MTX and PC, the MTX–PC complex was prepared by a co-solvent method between MTX and PC in reaction solvent (tetrahydrofuran, THF) for different reaction times (0, 0.5, 2, 4, 8, 12, and 24 h) (the obtained dispersion was photographed in Fig. S1†). Subsequently, the reaction solvent was removed by evaporation under reduced pressure. Dichloromethane (DCM) was added into the film followed by vigorous vortexing and the obtained dispersion was photographed (Fig. S2†). It was found that the MTX–PC complex dispersed in DCM gradually became clear and transparent as the reaction time increased, indicating the almost complete formulation between MTX and PC. Thus, we used 24 h as model reaction time for synthesis of the MTX–PC complex.
3.2. Characterization of MTX–PC complex
MTX is difficult to dissolve in DCM directly. Moreover, MTX could be precipitated in DCM after 24 h of storage (Fig. 3A). Furthermore, the solubility of MTX within DCM could not be obviously improved by simple physical mixing with PC. On the contrary, the prepared MTX–PC complex (Fig. S3†) is well-dispersed in THF or DCM and stable after 24 h and even 72 h of storage (Fig. 3A, 4, and S4†). The result indicated that the drug–phospholipid complex technique exerted the solubilization effect of MTX (the ultraviolet-visible (UV-vis) spectrum was seen in Fig. 3B). The complexate rate of MTX with PC in MTX–PC complex was determined as high as 92.5 ± 1.7% by high performance liquid chromatography (HPLC) analysis. Thus, it is inferred that when the non-polar solvent was introduced into the MTX–PC complex, the polar head of PC was oriented inward to MTX drug whereas the non-polar tail of PC was oriented outward to the organic phase, yielding the nearly spherical reversed micelles (TEM image was seen in Fig. 3C). We then used MTX, PC, physical mixture of MTX and PC, MTX–PC complex by differential scanning calorimetry (DSC), thermal gravity analysis (TGA), X-ray diffractometry (XRD), Fourier transform infrared spectroscopy (FT-IR), proton (1H)-nuclear magnetic resonance spectroscopy (NMR), and confocal laser scanning microscopy (CLSM) to investigate the distribution state of MTX drug in the pharmaceutical MTX–PC complex matrix. | ||
| Fig. 4 Optical image of MTX, PC, physical mixture of MTX and PC, and MTX–PC complex dispersed in THF for 72 h of storage. | ||
For DSC, the DSC curves of MTX showed two peaks (78 and 258 °C) (Fig. 3D). The DSC curves of the physical mixture demonstrated an additive effect of MTX and PC. With respect to the DSC curves of MTX, PC, and physical mixture, the original peaks of MTX disappeared in the MTX–PC complex. In addition, the similar result was also showed in the TGA curves of MTX, PC, their physical mixture, and MTX–PC complex (Fig. 3E). The result indicated that MTX interacted with PC by some weak molecular interactions in the MTX–PC complex.36,37
For XRD, the XRD pattern of MTX and PC respectively exhibited some sharp crystalline peaks and a broad peak (Fig. 3F), indicating a crystalline form of MTX and an amorphous one of PC. Moreover, the XRD pattern of the physical mixture clearly showed that some crystalline peaks were still detectable, indicating that MTX acted as a crystalline form remained in their physical mixture. On the contrary, all of crystalline peaks disappeared in the XRD pattern of the MTX–PC complex. The result revealed that MTX homogeneously dispersed in the PC matrix.
For FT-IR, the FT-IR spectrum of the physical mixture of MTX and PC showed an additive effect compared to that of MTX or PC (Fig. 5A). Whereas, the N–H stretching vibration of MTX at 3415 cm−1 shifted to a higher wavenumber at 3432 cm−1 in the FT-IR spectrum of the MTX–PC complex compared to that of MTX. The P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of PC at 1241 cm−1 also shifted to a higher wavenumber at 1254 cm−1 in the FT-IR spectrum of the MTX–PC complex compared to that of PC. It could be explained that the amino group of MTX may interact with the phosphate group of PC by electrostatic interactions in the structure formation of the MTX–PC complex. In addition, the absorption peaks of aromatic groups of MTX at 1049, 881, 804 cm−1 ascribed to the aromatic vibration almost disappeared in the spectrum of the MTX–PC complex, and the absorption peaks of quaternary amine group of PC at 970 cm−1 shifted to a lower wavenumber at 965 cm−1 in the spectrum of the MTX–PC complex, implying the presence of hydrogen bonds interaction between MTX and PC. On the other hand, the absorption peak of non-polar group of PC with two long fatty acid tails at 2925, 2854, 1736 cm−1 corresponding to C–H and C
O stretching vibration of PC at 1241 cm−1 also shifted to a higher wavenumber at 1254 cm−1 in the FT-IR spectrum of the MTX–PC complex compared to that of PC. It could be explained that the amino group of MTX may interact with the phosphate group of PC by electrostatic interactions in the structure formation of the MTX–PC complex. In addition, the absorption peaks of aromatic groups of MTX at 1049, 881, 804 cm−1 ascribed to the aromatic vibration almost disappeared in the spectrum of the MTX–PC complex, and the absorption peaks of quaternary amine group of PC at 970 cm−1 shifted to a lower wavenumber at 965 cm−1 in the spectrum of the MTX–PC complex, implying the presence of hydrogen bonds interaction between MTX and PC. On the other hand, the absorption peak of non-polar group of PC with two long fatty acid tails at 2925, 2854, 1736 cm−1 corresponding to C–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration was not associated to any change in the spectrum of the MTX–PC complex,20 suggesting that the long-chain fatty acids were not participated in formulation of the MTX–PC complex. Moreover, no new significant peaks appeared in the complex.
O stretching vibration was not associated to any change in the spectrum of the MTX–PC complex,20 suggesting that the long-chain fatty acids were not participated in formulation of the MTX–PC complex. Moreover, no new significant peaks appeared in the complex.
For 1H NMR, the NMR spectrum of the physical mixture of MTX and PC showed an additive effect of their individual components (Fig. 5B). Whereas, the majority of proton signals of MTX and a proton signal (∼3.5 ppm) of PC polar segment (phosphatidylcholine group) greatly suppressed or even disappeared in the NMR spectrum of the MTX–PC complex. In addition, the majority of the proton signals of PC non-polar segment were still detected in the NMR spectrum of the MTX–PC complex. The result might indicated that the weak molecular interactions between MTX and the polar head of PC resulted in the free rotate of outer non-polar tail of PC which enwrapped the inner polar head of PC with MTX.17
For CLSM, the green and red images were observed based on the DiD-labeled FITC–MTX–PC complex (MTX drug was labeled with FITC, PC material was labeled with DiD) using the green-excitated channel and red-excitated channel respectively. Both the green and red regions were completely overlapped with uniform yellow (Fig. 5C). The result indicated that the MTX drug molecules were uniformly distributed throughout the PC material matrix without phase separation.
Taken together, these results demonstrated the existence of multiple weak molecular interactions between MTX and polar parts of PC, including electrostatic interactions, hydrogen bond interactions, singly or in combination, which led to the highly effective formulation of the MTX–PC complex rather than just a simple physical mixture because of no generation of new peaks.16,17 Moreover, the non-polar part of PC was not participated in their complexation and turned freely to enwrap the polar parts to self-organized into the reverse micelles, which increased the lipophilicity of MTX essential for the subsequent self-assembly of amphiphilic MTX–PC complex.16,18
3.3. Characterization of MTX–PC complex self-assembled nanoparticles
Because of the amphiphilicity of MTX–PC complex, it can be self-assembled into micellar structures in water environment. The tyndall effect of the micellar dispersion under a laser beam provides the first indication for the formation of self-assembled nanoparticles (inset of Fig. 6A). Dynamic light scattering (DLS), electrophoretic light scattering (ELS), scanning electron microscopy (SEM), transmission electron microscopy (TEM), atomic force microscopy (AFM) were then used to investigate the self-assembly of MTX–PC complex. The hydrodynamic particle size of the MTX–PC NPs was 152.5 ± 3.2 nm with the polydispersity index (PDI) of 0.162 ± 0.015 determined using DLS (Fig. 6A), which is useful for cancer therapy via enhanced penetration and retention (EPR) effect, ensuring the passive tumor targeting mechanism.38,39 The surface charge of the MTX–PC NPs was −20.3 ± 2.1 mV (Fig. 6B), which would be helpful for stabilizing the NPs, decreasing the nonspecific interactions with negative charged red blood cells and serum proteins.40 In addition, the MTX–PC complex self-assembled nanoparticles showed no significant hydrodynamic particle size variation with the change of nanoparticles concentration (Fig. S5†). SEM, TEM, AFM images showed that the morphology of the MTX–PC NPs in Milli-Q water presented a spherical particle shape with a homogeneous particle distribution (Fig. 6C–F), and the particle size was in well agreement with the DLS result. In addition, the MTX–PC NPs also exhibited the excellent stability with no significant particle size change in aqueous media including Milli-Q water, phosphate buffer saline (PBS), and 10% fatal bovine serum (FBS) for 48 h (Fig. 6G).Most of the MTX targeting ligand/anticancer drug molecules was introduced within the PC complex by multiple weak molecular interactions and subsequently assembled into the NPs system with amorphous form (XRD result was seen in inset of Fig. 6D), which could be expected to realize the high drug incorporation efficiency, low drug burst release, and excellent drug targeting effect (discussed below). The nanoscaled particle size, narrow particle size distribution, negative surface charge, compact structure, and approximate stability suggested that the MTX–PC NPs are potential nanoscaled drug delivery systems for clinical use.22,41
3.4. Drug loading capacity
MTX interacted with phospholipids between its active groups and the phospholipid polar groups to yield the amphiphilic MTX–PC, thus leading to high drug loading capacity. The drug loading content of the MTX–PC NPs was determined as high as 20.7 ± 2.4%. Multiple MTX–PC molecules orderly arranged to formulate the core domains of the MTX–PC NPs. The extra MTX–PC acted as surfactants transferred to the surface of the MTX–PC NPs to sterically stabilize the nanoscaled system due to the surface energy reduction.423.5. In vitro drug release
The MTX release profile from the MTX–PC NPs was investigated by a dialysis method at pH 7.4 at 37 °C. Compared with the free MTX or MTX–PC liposomes prepared by a thin film hydration method,43 the MTX–PC NPs exhibited a reduced burst release followed by a more sustained release (Fig. 6H). A more sustained drug release of the pharmaceutical nanoparticles self-assembled by MTX–PC complex might be driven by a diffusion-controlled mechanism: MTX was dissociated from the MTX–PC complex uniformly distributed within the NPs first, and then MTX diffused from the NPs to outer medium.40,44 Compared to the core–shell capsuled structure of liposome, the self-assembled MTX–PC nanoparticles made it possible to achieve more better sustained drug release, so that more sustained therapeutic effects and lower toxic or side effect caused by burst drug release would appear. Both dug-loading and drug release results indicated that drug–phopholipid complex not only acted as an effective platform of drug loading but also served as a physical barrier to drug release.The pH of tumor tissue is much lower than normal tissue as a result of lactic acid produced by hypoxia and acidic intracellular organelles.23 This is very important for biological stimulus-mediated targeted drug delivery.26,27 Thus, the MTX release profile of the MTX–PC NPs was also investigated at pH 7.4, 6.5, and 5.0 at 37 °C, which respectively mimicked the physiological pH in blood circulation and normal tissues, the mildly acidic pH in tumor microenvironment, and the acidic pH in intracellular endo/lysosomes. It was found that MTX release from the MTX–PC NPs was much lower at pH 7.4 (approximately 60%) than at pH 5.0 (nearly 90%) over a 48 h period (Fig. 6I). This result was likely attributed to the ionization of MTX.45 As the external pH is decreased, the amino groups in MTX (see Fig. 1B) would ionize, leading to a rapid MTX release and thus a morphological change (TEM images were seen in Fig. S6†). In addition, the morphology transition was not conflicting with our goal since the loaded MTX could be sustainedly and controlledly released to exert the therapeutic activity.
3.6. In vitro cellular uptake
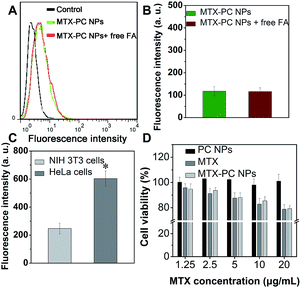
To address the selective cellular uptake efficacy of the MTX–PC NPs for FA receptors, we performed a competition assay on human cervical cancer cell lines HeLa cells with a high overexpression of FA receptors. HeLa cells were pretreated with excessive free FA for 15 min, then incubated with the DiD encapsulated MTX–PC NPs (MTX–PC NPs@DiD) for 4 h, and detected by confocal laser scanning microscopy. It was found that much enhanced red fluorescence intensity was clearly observed in FA-treated HeLa cells after incubated with the MTX–PC NPs@DiD compared to FA-untreated cells (Fig. 7A). The result indicated that the MTX–PC NPs were internalized by HeLa cells through FA receptors-mediated endocytosis. The similar result was also observed in another type of breast cancer cell lines MCF-7 cells (Fig. 7A), which highly overexpressed FA receptors. These results revealed the high-affinity recognization between MTX–PC NPs surface's multivalent MTX and HeLa or MCF-7 cells membrane surface which can greatly self-help the anticancer drug–phospholipid complex self-assembled NPs to target FA receptors-overexpressing cancer cells.3.7. Flow cytometry analysis
We next used a flow cytometer to analyze the cellular uptake efficiency of the MTX–PC NPs. The mean fluorescence intensities of FA-untreated HeLa cells after incubation with the MTX–PC NPs@DiD were much higher than those of FA-treated HeLa cells (Fig. 7B and C). In addition, it was found that the decreased mean fluorescence intensities of FA-treated HeLa cells incubated with the MTX–PC NPs@DiD were detected with increasing concentration of free FA (Fig. S7†). The difference in mean fluorescence intensities suggested that the targeting moiety offered by the MTX surface-functionalized drug–phospholipid complex self-assembled NPs was efficient at enhancing the cellular uptake efficiency. Thus, both the qualitative confocal microscopy images and quantitative flow cytometer results proved the high active targeting efficiency of MTX–PC NPs to FA receptors-overexpressing cancer cells.3.8. Intracellular drug delivery
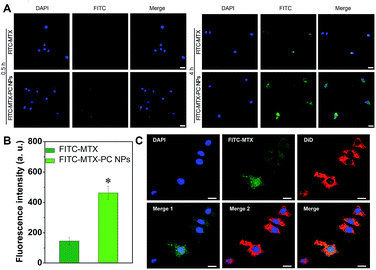
To investigate the intracellular MTX delivery of the MTX–PC NPs, we used FITC–MTX as a fluorescence probe to prepare the FITC–MTX–PC NPs and incubated HeLa cells with the FITC–MTX–PC NPs or free FITC–MTX. It was obviously found that the intracellular FITC–MTX delivered by both formulations increased as the incubation time was increased from 0.5 to 4 h. After 4 h of incubation, the stronger green fluorescence of HeLa cells treated with the FITC–MTX–PC NPs compared with the free FITC–MTX could be observed in HeLa cells (Fig. 8A). This result was also in line with the mean fluorescence intensities measured by flow cytometry analysis (Fig. 8B). These results demonstrated that the FITC–MTX–PC NPs entered cells more quickly than the free FITC–MTX. It was reported that FITC could not easily be internalized by cancer cells.34,46 Thus the difference was explained by their distinct cellular uptake mechanisms and endocytosis pathways. In addition, to confirm the cellular uptake and subsequent intracellular drug release, HeLa cells were treated with the FITC–MTX–PC NPs@DiD for 8 h. As shown in Fig. 8C, almost of red fluorescence of DiD was distributed in the cytoplasm because of FA receptor-mediated endocytosis of the MTX–PC NPs. By contrast, green fluorescence of FITC–MTX was observed in the cytoplasm and nucleus. Since it was impossible for the MTX–PC NPs to pass the nuclear pore complex of about 40 nm,47,48 this result could be caused by the release of MTX from the MTX–PC NPs. These results confirmed our hypothesis that the introduction of MTX into MTX-loaded drug–phospholipid complex self-assembled NPs can easily induce the FA receptors-mediated cellular uptake to elevate their intracellular drug concentration. Thus, the active targeting efficiency of MTX-loaded drug–phospholipid complex self-assembled NPs had great potentials to effectively kill FA receptors overexpressed tumor cells.3.9. In vitro cytotoxicity
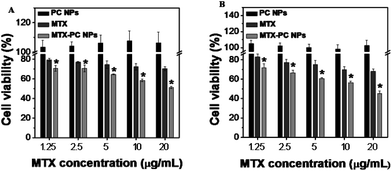
To investigate the cell-killing effect of the MTX–PC NPs, HeLa cells were treated with the MTX drug-free MTX–PC NPs (PC NPs), free MTX, and MTX–PC NPs for 24 h of incubation. The cell viability was evaluated using MTT assay. Compared to the free MTX, the MTX–PC NPs showed significantly enhanced cytotoxicity (Fig. 9A and B) towards HeLa cells and MCF-7 cells. The cytotoxicity of MTX–PC NPs and free MTX had statistics significance (P < 0.05). In other words, the free MTX need higher dosage to reach the same cell-killing efficiency than the MTX–PC NPs. Additionally, it was found that the PC NPs had no obvious cytotoxicity against HeLa cells and MCF-7 cells (Fig. 9A and B). This suggested that the phospholipid materials are safe and biocompatible for clinical use. Thus the results indicated the cytotoxicity of the MTX–PC complex-based NPs was induced by the released MTX and not by the drug-free phospholipid materials. These results combined with the intracellular drug delivery results (see Fig. 8) give us strong evidence that the multiple FA receptors-MTX ligands interactions can result in the active transport of the MTX–PC NPs loading MTX by FA receptor-mediated endocytosis. It should not be ignored that the lipophilic characteristic of MTX–PC complex-based self-assembled NPs could also facilitate the passive transport of MTX–PC NPs loading MTX drug from the water environment to the lipid-rich cell membrane.3.10. Interaction with normal cells
Mouse fibroblast cell lines NIH 3T3 cells with a low expression of FA receptors were utilized as normal cells to address the selectivity of the MTX–PC NPs between normal cells and cancer cells. It was found that both the FA-treated and FA-untreated NIH 3T3 cells exhibited no obvious difference in red fluorescent intensity after incubation with the MTX–PC NPs@DiD for 4 h (Fig. 10A and B). In addition, the stronger red fluorescent intensity was observed after the FA-untreated NIH 3T3 cells had been incubated with the MTX–PC NPs@DiD compared to the FA-untreated HeLa cells (Fig. 10C). These results indicated the presence of MTX on the surface of the MTX–PC NPs led to a lower cellular uptake by normal cells via non-specific endocytosis.It was noted that the MTX–PC NPs had much stronger cytotoxicity against cancer cells growth (see Fig. 9) but not normal cells (Fig. 10D) compared to the free MTX at the same concentration. It was well-known that majority of the free MTX entered tumor or normal cells via the reduced FA carrier but not membrane-associated FA receptor.24,31 Thus, the free MTX can result in the systemic toxicity due to its non-selectivity to tumor and normal cells, whereas the MTX–PC NPs with high selectivity can be engineered to deliver drug payload to tumor cells via membrane-associated FA receptor to reduce the detrimental side effect.
4. Conclusions
In our study, a simple and easy but successful method was developed to construct novel amphiphilic MTX (both a targeting ligand and an anticancer drug)–PC complex self-assembled NPs with improved formulation characteristics including smaller particle size, higher surface charge, and better stability. Compared to other nanoparticle system involved same drug and lipid like liposomes, the MTX–PC complex self-assembled NPs showed higher drug-loading content and more sustained drug release. In vitro cytotoxicity demonstrated that the MTX–PC NPs exhibited the significantly stronger and specific cytotoxicity than the free MTX. This work first revealed the high efficiency of self-targeting anticancer drug–phospholipid complex self-assembled nanoscaled drug delivery systems in both targeting and anticancer effect to FA receptors overexpressed cancer cells. Future work will be needed for in vivo study including biodistribution, pharmacokinetics, and anticancer activity of the MTX–PC NPs for possible clinical trials.Acknowledgements
This work was supported by the Natural Science Foundation of Fujian Province of China (Grant No. 2016J01406) and Natural Science Foundation of Jiangsu Province (Grant No. BK20130266).References
- R. L. Siegel, K. D. Miller and A. Jemal, Ca-Cancer J. Clin., 2015, 65, 5–29 CrossRef PubMed.
- S. Rizvi and G. J. Gores, Gastroenterology, 2013, 145, 1215–1229 CrossRef CAS PubMed.
- P. G. Rose, B. N. Bundy, E. B. Watkins, J. T. Thigpen, G. Deppe, M. A. Maiman, D. L. Clarke-Pearson and S. Insalaco, N. Engl. J. Med., 1999, 340, 1144–1153 CrossRef CAS PubMed.
- T.-C. Chou, Pharmacol. Rev., 2006, 58, 621–681 CrossRef CAS PubMed.
- B. A. Chabner and T. G. Roberts, Nat. Rev. Cancer, 2005, 5, 65–72 CrossRef CAS PubMed.
- C. J. Cheng, G. T. Tietjen, J. K. Saucier-Sawyer and W. M. Saltzman, Nat. Rev. Drug Discovery, 2015, 14, 239–247 CrossRef CAS PubMed.
- M. E. Davis, Z. G. Chen and D. M. Shin, Nat. Rev. Drug Discovery, 2008, 7, 771–782 CrossRef CAS PubMed.
- R. A. Petros and J. M. DeSimone, Nat. Rev. Drug Discovery, 2010, 9, 615–627 CrossRef CAS PubMed.
- H. A. Brown, Nat. Chem. Biol., 2008, 4, 581–582 CrossRef CAS PubMed.
- E. Frei, N. Jaffe, M. H. N. Tattersall, S. Pitman and L. Parker, N. Engl. J. Med., 1975, 292, 846–851 CrossRef PubMed.
- A. L. Jackman, D. S. Theti and D. D. Gibbs, Adv. Drug Delivery Rev., 2004, 56, 1111–1125 CrossRef CAS PubMed.
- D. A. Matthews, R. A. Alden, J. T. Bolin, S. T. Freer, R. Hamlin, N. Xuong, J. Kraut, M. Poe, M. Williams and K. Hoogsteen, Science, 1977, 197, 452–455 CAS.
- J. Jolivet, K. H. Cowan, G. A. Curt, N. J. Clendeninn and B. A. Chabner, N. Engl. J. Med., 1983, 309, 1094–1104 CrossRef CAS PubMed.
- Z. A. Khan, R. Tripathi and B. Mishra, Expert Opin. Drug Delivery, 2012, 9, 151–169 CrossRef CAS PubMed.
- J. Chen, L. Huang, H. Lai, C. Lu, M. Fang, Q. Zhang and X. Luo, Mol. Pharmaceutics, 2014, 11, 2213–2223 CrossRef CAS PubMed.
- J. Khan, A. Alexander, Ajazuddin, S. Saraf and S. Saraf, J. Controlled Release, 2013, 168, 50–60 CrossRef CAS PubMed.
- P. M. Kidd, Alternative Med. Rev., 2009, 14, 226–246 Search PubMed.
- F. Cui, K. Shi, L. Zhang, A. Tao and Y. Kawashima, J. Controlled Release, 2006, 114, 242–250 CrossRef CAS PubMed.
- F. Yu, Y. Li, Q. Chen, Y. He, H. Wang, L. Yang, S. Guo, Z. Meng, J. Cui, M. Xue and X. D. Chen, Eur. J. Pharm. Biopharm., 2016, 103, 136–148 CrossRef CAS PubMed.
- Z. Hou, Y. Li, Y. Huang, C. Zhou, J. Lin, Y. Wang, F. Cui, S. Zhou, M. Jia, S. Ye and Q. Zhang, Mol. Pharmaceutics, 2013, 10, 90–101 CrossRef CAS PubMed.
- Y. Li, J. Lin, X. Yang, Y. Li, S. Wu, Y. Huang, S. Ye, L. Xie, L. Dai and Z. Hou, ACS Appl. Mater. Interfaces, 2015, 7, 17573–17581 CAS.
- D. Peer, J. M. Karp, S. Hong, O. C. Farokhzad, R. Margalit and R. Langer, Nat. Nanotechnol., 2007, 2, 751–760 CrossRef CAS PubMed.
- F. Danhier, O. Feron and V. Preat, J. Controlled Release, 2010, 148, 135–146 CrossRef CAS PubMed.
- P. S. Low, W. A. Henne and D. D. Doorneweerd, Acc. Chem. Res., 2008, 41, 120–129 CrossRef CAS PubMed.
- S. Song, F. Chen, H. Qi, F. Li, T. Xin, J. Xu, T. Ye, N. Sheng, X. Yang and W. Pan, Pharm. Res., 2014, 31, 1032–1045 CrossRef CAS PubMed.
- S. R. MacEwan, D. J. Callahan and A. Chilkoti, Nanomedicine, 2010, 5, 793–806 CrossRef CAS PubMed.
- W. Hou, X. Zhao, X. Qian, F. Pan, C. Zhang, Y. Yang, J. M. De la Fuente and D. Cui, Nanoscale, 2016, 8, 104–116 RSC.
- Y. Li, J. Lin, H. Wu, M. Jia, C. Yuan, Y. Chang, Z. Hou and L. Dai, J. Mater. Chem. B, 2014, 2, 6534–6548 RSC.
- K. Mizusawa, Y. Takaoka and I. Hamachi, J. Am. Chem. Soc., 2012, 134, 13386–13395 CrossRef CAS PubMed.
- A. Qiu, M. Jansen, A. Sakaris, S. H. Min, S. Chattopadhyay, E. Tsai, C. Sandoval, R. Zhao, M. H. Akabas and I. D. Goldman, Cell, 2006, 127, 917–928 CrossRef CAS PubMed.
- S. Rijnboutt, G. Jansen, G. Posthuma, J. B. Hynes, J. H. Schornagel and G. J. Strous, J. Cell Biol., 1996, 132, 35–47 CrossRef CAS PubMed.
- J. X. Kelly, M. J. Smilkstein, R. Brun, S. Wittlin, R. A. Cooper, K. D. Lane, A. Janowsky, R. A. Johnson, R. A. Dodean, R. Winter, D. J. Hinrichs and M. K. Riscoe, Nature, 2009, 459, 270–273 CrossRef CAS PubMed.
- Y. Li, J. Lin, H. Wu, Y. Chang, C. Yuan, C. Liu, S. Wang, Z. Hou and L. Dai, Mol. Pharmaceutics, 2015, 12, 769–782 CrossRef CAS PubMed.
- H. Wang, Y. Zhao, Y. Wu, Y.-l. Hu, K. Nan, G. Nie and H. Chen, Biomaterials, 2011, 32, 8281–8290 CrossRef CAS PubMed.
- F. Yu, Y. Li, C. S. Liu, Q. Chen, G. H. Wang, W. Guo, X. E. Wu, D. H. Li, W. D. Wu and X. D. Chen, Int. J. Pharm., 2015, 484, 181–191 CrossRef CAS PubMed.
- M. Lucio, F. Bringezu, S. Reis, J. L. Lima and G. Brezesinski, Langmuir, 2008, 24, 4132–4139 CrossRef CAS PubMed.
- E. Lasonder and W. D. Weringa, J. Colloid Interface Sci., 1990, 139, 469–478 CrossRef CAS.
- V. Torchilin, Adv. Drug Delivery Rev., 2011, 63, 131–135 CrossRef CAS PubMed.
- J. Lin, Y. Li, Y. Li, H. Wu, F. Yu, S. Zhou, L. Xie, F. Luo, C. Lin and Z. Hou, ACS Appl. Mater. Interfaces, 2015, 7, 11908–11920 CAS.
- Y. Li, H. Wu, X. Yang, M. Jia, Y. Li, Y. Huang, J. Lin, S. Wu and Z. Hou, Mol. Pharmaceutics, 2014, 11, 2915–2927 CrossRef CAS PubMed.
- F. Yu, Y. Li, C. S. Liu, Q. Chen, G. H. Wang, W. Guo, X. E. Wu, D. H. Li, W. D. Wu and X. D. Chen, Int. J. Pharm., 2015, 484, 181–191 CrossRef CAS PubMed.
- J. Liu, T. Gong, C. Wang, Z. Zhong and Z. Zhang, Int. J. Pharm., 2007, 340, 153–162 CrossRef CAS PubMed.
- P. Srisuk, P. Thongnopnua, U. Raktanonchai and S. Kanokpanont, Int. J. Pharm., 2012, 427, 426–434 CrossRef CAS PubMed.
- G. Acharya and K. Park, Adv. Drug Delivery Rev., 2006, 58, 387–401 CrossRef CAS PubMed.
- H. Cheng, Y.-J. Cheng, S. Bhasin, J.-Y. Zhu, X.-D. Xu, R.-X. Zhuo and X.-Z. Zhang, Chem. Commun., 2015, 51, 6936–6939 RSC.
- Y. Li, H. Wu, M. Jia, F. Cui, J. Lin, X. Yang, Y. Wang, L. Dai and Z. Hou, Mol. Pharmaceutics, 2014, 11, 3017–3026 CrossRef CAS PubMed.
- X. Zhang, K. Achazi, D. Steinhilber, F. Kratz, J. Dernedde and R. Haag, J. Controlled Release, 2014, 174, 209–216 CrossRef CAS PubMed.
- E. Jin, B. Zhang, X. Sun, Z. Zhou, X. Ma, Q. Sun, J. Tang, Y. Shen, E. Van Kirk, W. J. Murdoch and M. Radosz, J. Am. Chem. Soc., 2013, 135, 933–940 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials and methods, general measurements, control experiments, additional table and figures as described in the word. See DOI: 10.1039/c6ra17260a |
| ‡ Yanxiu Li and Jinyan Lin contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |