Homogeneous sulphur-doped composites: porous carbon materials with unique hierarchical porous nanostructure for super-capacitor application
Abstract
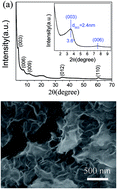
Specific surface area, hierarchical porosity and heteroatom doping have been proved to be desirable for carbon electrode materials in advanced super-capacitors. In this study, homogeneous sulphur-doped porous carbon materials (L-HPCs) with a unique hierarchical porous nanostructure have been prepared by the combination of the two-dimensional interlayer confinement effect of a layered double hydroxide (LDH) and KOH activation method. XRD, SEM, TEM, BET, EIS, cyclic voltammetry and the constant current charge–discharge test were used to investigate the morphology, structure, surface properties and electrochemical performances of the carbon materials. The L-HPCs fabricated by this method have a large specific surface area of 2154.0 m2 g−1 and contain plenty of micropores and mesopores, which arise from the carbonisation of organic polymers, the presence of LDH and the catalytic effect of iron in LDH layers in the calcination process. The L-HPCs serving as an electrode material for super-capacitors exhibited a specific capacitance as high as 259 F g−1 at 1 A g−1 in 6 M KOH electrolyte. This excellent electrochemical performance might be due to the high surface area, high pore volume and hierarchical porous structure of the carbon materials, which provide a quick ion transfer pathway to electrolyte access into the microporous area. At the same time, the doping of heteroatoms can improve the conductivity of the electrode materials.

 Please wait while we load your content...
Please wait while we load your content...