Thermal, mechanical and magnetic properties of functionalized magnetite/vinyl ester nanocomposites†
Abstract
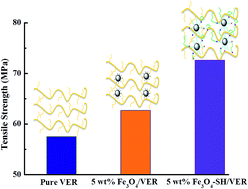
Magnetite (Fe3O4) nanoparticles grafted with thiol groups through silanization of (3-mercaptopropyl) trimethoxy-silane (Fe3O4-SH) were used for preparing vinyl ester resin (VER) nanocomposites with different Fe3O4 nanoparticle loading levels. The calculated thiol group graft percentage of only ∼2.25% did show a significant effect on the physicochemical properties of VER nanosuspensions and nanocomposites. The exothermal curing peak temperature was shifted from 80 °C for pure VER to 75 °C for liquid VER with 10 wt% Fe3O4-SH. The tensile strength (72.6 MPa) of 5 wt% Fe3O4-SH reinforced nanocomposites was increased by 26.3% as compared with that (57.5 MPa) of pure VER composites. However, the tensile strength (62.7 MPa) of 5 wt% Fe3O4 was just increased by 9.0%. The polymer matrix and surface coating were observed to have negligible effects on the magnetic properties of nanoparticles, exhibiting superparamagnetic behaviors.


 Please wait while we load your content...
Please wait while we load your content...