Reversible and easy post-crosslinking method for developing a surface ion-imprinted hypercrosslinked monolith for specific Cd(II) ion removal from aqueous solutions†
Mehmet Emin Çormanab,
Canan Armutcua,
Lokman Uzun*a,
Rıdvan Saycd and
Adil Denizlia
aHacettepe University, Faculty of Science, Department of Chemistry, 06381-Beytepe, Ankara, Turkey. E-mail: lokman@hacettepe.edu.tr; Fax: +90 312 299 2163; Tel: +90 312 297 7337
bSinop University, Department of Bioengineering, Sinop, Turkey
cAnadolu University, Department of Chemistry, Eskişehir, Turkey
dBionkit Ltd Şti, Eskişehir, Turkey
First published on 8th September 2016
Abstract
In this study, a new surface imprinting technique for preparing a hypercrosslinked monolith to remove Cd(II) ions out from aqueous solutions was proposed. The monoliths were characterized by Fourier transform infrared spectroscopy, scanning electron microscopy, thermal gravimetrical analysis, surface area measurements and elemental analysis. The reversible nature of the hypercrosslinking process was proved by repeated crosslinking and denaturation cycles by using ferric ions as an oxidant and urea as a reductant, respectively meanwhile performing surface area measurements for both situations to demonstrate the variation in the surface porosity. The multipoint BET surface areas of poly(HEMA), surface ion imprinted (Cd–SII-HM) and non-imprinted (NI-HM) monoliths were determined as 269.1 m2 g−1, 79.1 m2 g−1, and 67.4 m2 g−1, respectively. By breaking hypercrosslinks, the micropore volume decreased from 39.7 mm3 g−1 to 11.8 mm3 g−1 while the cumulative pore volume decreased from 30.7 mm3 g−1 to 9.1 mm3 g−1 during urea treatment. At the first step, the affecting factors such as initial Cd(II) ion concentrations, pH and adsorption time were optimized. Then, the selectivity of the Cd–SII-HM for Cd(II) against other metal ions was evaluated not only from singular solutions but also from triple and quadruple solutions, which included Pb(II), Zn(II) and Hg(II) ions as competitors. The relative selectivity coefficients were calculated as 3.28, 15.61 and 58.55 for Cd(II)/Zn(II), Cd(II)/Pb(II) and Cd(II)/Hg(II) pairs. The results obtained indicated that the developed reversible and easy post-crosslinking method is quite applicable for producing the surface ion-imprinted polymers with high selectivity for the template ions [Cd(II)] regarding the potential competitor ions [Zn(II), Pb(II) and Hg(II)].
1. Introduction
Growing population, urbanization, and the rapidly developing industrialization, have also brought about problems of environmental pollution since the beginning of the twentieth century. Heavy metal pollution has become one of today's most critical environmental cases.1,2 Heavy metals are both resistant to environmental conditions and don't turn into safe products with biological degradation so these can cause severe damage to humans and other living organisms. Cadmium ions [Cd(II)] are among the top-ten toxic heavy metals in the most recently reported priority hazardous substance list of the Agency for Toxic Substances and Disease Registry (ATSDR) in 2013.3 Also, International Agency for Research on Cancer (IARC) classified cadmium as a carcinogen (group IA).4 With the industrialization and industrial development, high concentrations of cadmium can occur in wastewater. Accumulation of cadmium in water firstly affect living organisms in the aquatic area and many species such as plants, animals and humans are exposed to that.5 Cadmium has become a cumulative toxin because of the extremely long biological half-life in human bodies. When cadmium is accumulated in the body, it can cause severe damage to different organs such as kidneys, liver, lungs, pancreas, testes, placenta, cardiovascular, skeletal system, nervous system, immune and reproductive systems.6,7 Because of these harmful effects, removal of cadmium from wastewater is enormously important. For this purpose, many methods such as chemical precipitation, ion exchange, solid-phase extraction have been employed for cadmium removal.8 In addition to these methods, nowadays, surface imprinting technique has been used for selective removal of cadmium ions.Molecular imprinting is the one of the developing multidimensional approaches for creating the highly specific polymeric materials.9 For this purpose, a pre-assembly step is constituted by using the functional monomers and template molecules before polymerization in the presence of the crosslinker. Subsequently, the removal of the template molecules from polymerization network exposed the molecular cavities that fully fitted to the molecule of the interest. Similar to the molecularly imprinted polymers (MIPs), ion-imprinted polymers (IIPs) are prepared via the same approach except using an ion as the template.10 Preparation of IIPs by conventional imprinting technique have some disadvantages such as thick imprinted polymers, fewer recognition sites, limited binding capacity, low binding kinetic and slow mass transfer because template molecules might be imprinted to the deep in the matrices.11 To overcome these handicaps, a novel and advanced imprinting technique called surface molecular imprinting has been developed in recent years.12–14 This approach provides more selective recognition sites due to that the imprinting occurred on the polymer surface as well as much more efficient accessibility to the cavities of template molecules occur.15 Because of these attractive features, the researchers utilized the surface imprinted polymers to develop the novel materials having both fast mass transfer and high affinity.16,17
Hypercrosslinked polymers are a class of polymeric materials which are highly crosslinked macromolecules due to the three-dimensional tree-like structure.18,19 Hypercrosslinked polymers have a high efficiency through network structures and also their unique physical and chemical properties, which made them as a popular research subject. The randomly crosslinked polymers are polydispersed and synthesized via one-step polymerization reactions, and so cost-friendly product that can be prepared easily.19,20 In recent years, multi-step polymerization techniques have been conducted to produce hypercrosslinked polymers for widely using in coatings, drug delivery systems, environmental protection, dye industry, supramolecular chemistry and so forth application fields because of these unique properties.19,21 Monoliths are continuous functional materials that have interconnected different pore structure which allows a facilitated convective mass transfer.22 Besides these large pores, the meso- and micro-pores ensure active binding sites for molecules of interest.23,24 All pores form a network channel in monoliths; so monoliths provide high flow rates, low back pressure, and high loading capacity.24 Also, the diffusion is not limiting process due to efficient mass transfer using convective flow between mobile and stationary phases.25,26
In this study, we proposed a novel approach of surface ion-imprinted hypercrosslinked monolith for an efficient Cd(II) ions removal from aqueous solutions by combining of the monolith, hypercrosslinking and surface imprinting approaches into a single study to create a synergy between useful research fields. For this purpose, the polymerizable derivative of cysteine amino acid (N-methacryloyl-L-cysteine, MAC) was utilized for creating surface ion-imprinted hypercrosslinked monoliths (Cd–SII-HM). Before the removal studies, the Cd–SII-HMs were characterized by applying Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), thermal gravimetric analysis (TGA), surface area measurements and elemental analysis. Then, Cd(II) ions removal studies were performed from aqueous solutions by evaluating the effective parameters including initial concentration, pH and adsorption time on the Cd(II) ions recognition capability. The reversibility of the hypercrosslinking process was confirmed by conducting crosslinking/denaturing cycles with ferric ions as oxidant and urea as reductant, respectively. Finally, the selectivity of the Cd–SII-HMs was evaluated against Cd(II) ions in regarding the potential competitor ions such as Pb(II), Zn(II) and Hg(II) ions from not only singular but also triple and quadruple solutions.
2. Materials and methods
2.1. Materials
The essential monomer, 2-hydroxyethyl methacrylate (HEMA), cross-linker, ethylene glycol dimethacrylate (EGDMA), initiator, azobis(isobutyronitrile) (AIBN), pore-former (toluene), ethylenediamine tetraacetic acid (EDTA), hydroquinone, FeCl3, L-Cysteine and methacryloyl chloride were purchased from Sigma-Aldrich (St. Louis, MO, USA). The solutions used in adsorption and selectivity studies of Cd(II), Pb(II), Zn(II) and Hg(II) metal ions were prepared from their nitrate salts purchased from Merck AG (Darmstadt, Germany). All of the water was purified using a Barnstead ROpure LP reverse osmosis unit (Dubuque, IA, USA) to use in the adsorption experiments. All glasswares were washed with a dilute nitric acid solution and deionized water, respectively; then dried them in an oven under dust-free conditions before use.2.2. Preparation of the monoliths
In this study, we synthesized the hypercrosslinked monoliths (both the surface imprinted and non-imprinted) through the in situ bulk polymerization. For this aim, the functional monomer, N-methacryloyl-L-cysteine (MAC) was firstly synthesized according to our previous publications.27,28 The MAC (20 mg dissolved in 0.25 mL of deionized water), HEMA (4 mL), hydroquinone (12 mg) and AIBN (30 mg) were mixed in toluene (8 mL). Then, this mixture was put into a screw-capped glass reactor and magnetically stirred at 65 °C for 2 h. After this first-step polymerization to convert functional monomers into the linear polymeric chain having the functional (thiol) groups, the crosslinker (EGDMA, 1 mL) and additional diluent (toluene, 1 mL) were added and subsequently kept the mixture at 65 °C for 1 h while magnetically vigorous stirring. Then, the slightly crosslinked pre-polymer solutions were poured into the syringes with a closed outlet and kept them at 65 °C for 1 h and 85 °C for 2 h for further chain extension and termination of the polymerization. After the polymerization step, the syringes were cooled down to room temperature and extensively washed the monoliths to remove out the unreacted monomers and the diluent. Hereafter, the monolith was treated with Cd(II) ion solution that prepared at the MAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cd(II) ratio as 5
Cd(II) ratio as 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by mole for 4 h in the case of surface imprinted monolith. After that, the ferric ion solution was passed through the monoliths [both Cd(II) treated and untreated] to create the –S–S– bridges between free thiol groups. Herein, the ferric ion solution used was prepared from FeCl3 salt in regarding the MAC
1 by mole for 4 h in the case of surface imprinted monolith. After that, the ferric ion solution was passed through the monoliths [both Cd(II) treated and untreated] to create the –S–S– bridges between free thiol groups. Herein, the ferric ion solution used was prepared from FeCl3 salt in regarding the MAC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe(III) ratio as 1
Fe(III) ratio as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.6 by mole. The Cd(II) treated monolith was designated as Cd(II) surface ion imprinted hypercrosslinked monolith (Cd–SII-HM) whereas untreated one was designated as the non-imprinted hypercrosslinked monolith (NI-HM). The elution of the template ions from the polymeric network exposed the imprinted cavities, which was achieved by treating Cd–SII-HM with 50 mM EDTA for 24 h. Finally, both monoliths were washed with 0.1 HNO3 (10 mL) to ensure the complete removal of the template and cleaning of the polymeric network.
1.6 by mole. The Cd(II) treated monolith was designated as Cd(II) surface ion imprinted hypercrosslinked monolith (Cd–SII-HM) whereas untreated one was designated as the non-imprinted hypercrosslinked monolith (NI-HM). The elution of the template ions from the polymeric network exposed the imprinted cavities, which was achieved by treating Cd–SII-HM with 50 mM EDTA for 24 h. Finally, both monoliths were washed with 0.1 HNO3 (10 mL) to ensure the complete removal of the template and cleaning of the polymeric network.
2.3. Characterization of the monoliths
Both surface imprinted (Cd–SII-HM) and non-imprinted (NI-HM) hypercrosslinked monoliths were characterized by using FTIR-ATR, SEM, TGA, surface area measurements and elemental analysis. SEM was used to visualize the physical appearance of both of Cd–SII-HM and NI-HMs after completely drying them at room temperature. The one piece of polymer from the samples was mounted on the holder to be coated with a thin gold layer for 2 min. Then, the samples were examined to take images at different magnifications by using SEM instrument (JEOL, JEM 1200EX, Tokyo, Japan).FTIR spectroscopy was conducted to analyze the chemical structures of both of Cd–SII-HM and NI-HMs by using FTIR-ATR spectrophotometer (Thermo Fisher Scientific, Nicolet iS10, Waltham, MA, USA). Herein, the plain polymer [poly(2-hydroxyethyl methacrylate)] were also analyzed for comparison purpose. Two grams of monolith sample which was dried and crushed into fine particles was mixed with potassium bromide (98 mg, KBr, IR-grade) to form pellets for FTIR analyses in the wavenumber range of 4000–650 cm−1.
The thermal gravimetrical (TGA) and elemental analyses (EA) were conducted to confirm and quantify the incorporation of functional monomer (MAC). TGA was performed by using the analyzer (Shimadzu DTG-60, Tokyo, Japan) at a heating rate 10 °C min−1 in the temperature range of 30°C–500 °C whereas EA by using the analyzer (Leco, CHNS-932, USA) with respect to sulfur stoichiometry. Finally, multipoint Brunauer–Emmett–Teller (BET) analysis was applied to assess the specific surface area variation during hypercrosslinking and denaturation by using nitrogen as an adsorbate gas.
2.4. Adsorption experiments
The adsorption experiments were carried out with both Cd–SII-HM and NI-HMs in a continuous adsorption setup by using a peristaltic pump (Watson-Marlow, Wilmington, MA, USA) to optimize the Cd(II) ion interaction conditions. Herein, the effects of pH, initial Cd(II) concentration and adsorption time were evaluated. Firstly, the Cd(II) ion adsorption studies at different pH values were performed in the range of 3.0–7.0, which was adjusted with 0.1 M HNO3 and 0.1 M NaOH solutions prepared in deionized water. After that, the initial Cd(II) ion concentration (in the range of 5–500 ppm) and adsorption time (in the range of 5–120 min) were evaluated at predetermined pH value. During all adsorption studies, the concentration of the metal ions in aqueous solutions was measured using a flame atomic absorption spectrophotometer (FAAS; AAnalyst 800, PerkinElmer, USA). Meantime, the instrument response was periodically checked and standardized for each metal ion after the measurements. All metal ion experiment (n = 3) and FAAS measurements (n = 3) were triplicated, and the calculations were applied within the 95% confidence interval.2.5. The selectivity studies
The selectivity of Cd–SII-HMs was evaluated towards Cd(II) ions (ionic radius: 114 pm) in accordance to Pb(II) (ionic radius: 133 pm), Zn(II) (ionic radius: 88 pm) and Hg(II) (ionic radius: 116 pm) ions. The selection criteria for these ions are not only ionic radius but also having affinity similar to Cd(II) ions. Herein, adsorption studies were performed under three different conditions: (i) from singular heavy metal ion solutions (uncompetitive); (ii) triple mixture of Cd(II), Zn(II) and Pb(II) ions in 25 ppm initial concentration for each (competitive 1); and (iii) quadruple mixture of Cd(II), Zn(II), Pb(II) and Hg(II) ions in 25 ppm initial concentration (competitive 2). By this way, the selectivity variation of the materials developed was investigated under whether competitive or uncompetitive conditions. It should also be mentioned that all experiments were simultaneously carried out by using NI-HMs under same conditions for comparison purpose. Finally, the fundamental coefficients including distribution (Kd), selectivity (k) and relative selectivity (k′) coefficients for both monoliths while considering the data for all ions, Cd(II), Pb(II), Zn(II) and Hg(II) were calculated by using the following mathematical formulas:| Kd = [(Ci − Cf)/Cf] × V/m | (1) |
| k = Kd,template/Kd,competitor | (2) |
| k′ = KCd–SII-MH/kNI-MH | (3) |
Desorption of adsorbed metal ions was carried out using 50 mM EDTA in a continuous system and was also determined by FAAS. Also, the reusability of Cd–SII-HMs was evaluated by performing ten-successive Cd(II) metal ion adsorption–desorption cycles to draw a project of the operational cost of the system.
2.6. Preparation of real samples
The tap water samples were obtained from a local supplier (Beytepe, Ankara, Turkey). The synthetic seawater was prepared according to literature.28 All the water samples were filtered through a 0.45 μM membrane filter before use. These water samples were spiked with two concentrations (5 and 10 ppm) of Cd(II) ions. Real samples experiments were carried out with Cd–SII-HM as mentioned before under optimized adsorption conditions.3. Results and discussion
3.1. The synthesis and characterization of the monoliths
The hypercrosslinked monoliths were synthesized by following two-step polymerization approach (Scheme 1). The characteristic bands were obtained in the FTIR spectra of Cd–SII-HM and NI-HMs as stretching vibration band of hydrogen bonded alcohol (–O–H), carbonyl (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), amide I/II (–CONH–), and aliphatic C–H bands, around 3442 cm−1, 1728 cm−1, 1640/1483 cm−1, and 2954 cm−1, respectively. In addition to these shared bands, Cd–SII-HMs have ester (–COO–) bands and the characteristic strong S–H stretching bands at, 1456/1390 cm−1 and 903 cm−1, respectively (Fig. 1). Also, surface morphologies and internal structure of the monoliths were examined by using SEM (Fig. 2). According to the figure, both of the monoliths have interconnected porous structure having a different pore size and very rough surfaces. As also seen in the figure, the diameter range of pores varied in the range of 2–20 μm, which indicated highly porous nature of the monoliths with a reduced diffusional mass transfer resistance and a facilitated convective mass transfer. Because of these attractive features, metal ions flow readily through the monoliths and high metal ion adsorption capacities were provided.
O), amide I/II (–CONH–), and aliphatic C–H bands, around 3442 cm−1, 1728 cm−1, 1640/1483 cm−1, and 2954 cm−1, respectively. In addition to these shared bands, Cd–SII-HMs have ester (–COO–) bands and the characteristic strong S–H stretching bands at, 1456/1390 cm−1 and 903 cm−1, respectively (Fig. 1). Also, surface morphologies and internal structure of the monoliths were examined by using SEM (Fig. 2). According to the figure, both of the monoliths have interconnected porous structure having a different pore size and very rough surfaces. As also seen in the figure, the diameter range of pores varied in the range of 2–20 μm, which indicated highly porous nature of the monoliths with a reduced diffusional mass transfer resistance and a facilitated convective mass transfer. Because of these attractive features, metal ions flow readily through the monoliths and high metal ion adsorption capacities were provided.
The functional monomer (MAC) incorporated into polymeric backbone was also characterized and quantified by applying elemental and thermal gravimetrical analyses. The MAC content of the monoliths was determined as 45 μmol g−1 monolith. The elemental analysis was conducted to plain polymers [poly(HEMA)] to ensure the origin of the sulfur stemmed from the functional monomer. Fig. 3 showed the thermal stability of the monoliths in the temperature range of 20–460 °C under a nitrogen atmosphere. As seen in the figure, there is five different decomposition zones: (i) 25–150 °C, (ii) 150–240 °C, (iii) 240–300 °C, (iv) 300–360 °C and (v) 360–410 °C and its beyond. First temperature zone, the dehydration of polymeric network, is corporate for four different samples that were the plain polymer [poly(HEMA)], Cd–SII-HM, Cd–SII-HM* [after Cd(II) ions removal] and NI-HM as expected. The second zone depended on the removal of polar –OH groups from the polymeric network; therefore, all samples had a decomposition peaks in this region as well. The third zone was related to the decarboxylation of the polymeric chains, which was the latest corporate zone for all samples. After this zone, poly(HEMA) had no decomposition peak because of the absence of polar functional group in the polymeric chain except carbonization at high temperatures (after 420 °C). NI-HM had one more peak in the fourth zone, which was related to the decomposition of the oxidized form of the functional tail (–S–S–). For Cd–SII-HMs, there is two more peaks in the zone 4 and 5; the previous one was related to the decomposition of free polar –SH groups whereas latest one was related to the decomposition of the oxidized form of the functional tail (–S–S–), respectively. Moreover, the presence of template ions [Cd(II)] decreased the decomposition rate (blue curve) while the ions acted as a crosslinker and penetrated/delayed the decomposition of free –SH groups (black curve). The Cd–SII-HM* has only free –SH groups, which confirmed the surface imprinted cavities, therefore it had clear decomposition peak in the temperature zone 5. At higher temperatures, there is slight weight loss due to the carbonization of polymeric chains.
 | ||
| Fig. 3 The TGA thermograms of the monoliths. The heating rate: 10 °C min−1 and temperature range: 30 °C–500 °C. | ||
The specific surface areas of the monoliths were determined via multipoint Brunauer–Emmett–Teller (BET) analysis to follow the variation during a reversible hypercrosslinking/denaturation cycle (ESI, Fig. SI-1–8†). For this aim, ferric ions were used as an oxidant and urea as a reductant. The multipoint BET surface areas of poly(HEMA), Cd–SII-HM and NI-HMs were 269.1 m2 g−1, 79.1 m2 g−1, and 67.4 m2 g−1, respectively. The significant difference between the plain and functional monoliths indicated the incorporation of functional monomers into the polymeric structure and the formation of functional tails which would act in the ion coordination and crosslinking process. After urea treatment to break down the –S–S– bridges, the values decreased to 23.5 m2 g−1 and 31.2 m2 g−1 for Cd–SII-HM and NI-HM, respectively. These results confirmed the reduction of hypercrosslinked bridges and the reversible nature of the crosslinking process. The micropore area decreased from 111.4 m2 g−1 to 33.1 m2 g−1 while the micropore volume decreased from 39.7 mm3 g−1 and 11.8 mm3 g−1. Also, micropore volume decreased from 39.7 mm3 g−1 to 11.8 mm3 g−1 while cumulative pore volume decreased from 30.7 mm3 g−1 to 9.1 mm3 g−1 during urea treatment. These results clearly revealed the breaking of the –S–S– bridges and the destruction of Cd(II) imprinted cavities on the surface of the monolithic polymeric chains. As a conclusion, the results showed that the reversible hypercrosslinking and the surface ion imprinting process were easily achieved by the urea and ferric ion treatments.
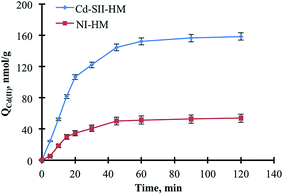
3.2. Optimization of Cd(II) ion adsorption
3.3. Selectivity studies
The main aim to create imprinted polymers is to introduce the selective recognition capability into the polymeric network; so imprinted polymers are called as plastic antibodies as well.31 Therefore, the selectivity of Cd–SII-HMs was demonstrated against the template ions [Cd(II)] thoroughly three different approaches: the adsorption studies from (i) singular, (ii) triple and (iii) quadruple solutions in the presence/absence of heavy metal ions [Zn(II), Pb(II) and Hg(II)] as the potential competitors. The selection criteria for this stage are ionic radii of heavy metal ions and similar affinity to the functional groups. Pb(II) and Hg(II) ions, (respectively 133 and 116 pm) have larger ionic radii whereas the Zn(II) ions (88 pm) have smaller ionic radius than that Cd(II) ions have (114 pm).32 In all three cases, Cd–SII-HMs have the highest affinity against the template Cd(II) ions whereas NI-HMs have a variation in the affinity order for the heavy metal ions. However, the adsorption capacities for all ions decreased in the cases of all three experiment setups due to the antagonistic effects of the heavy metal ions which indicated the competition between them (Table 1). Also, the selectivity coefficients for Cd–SII-HMs were quite high (between 5.11 and 53.47) for all cases whereas those for NI-HMs were relatively low (between 0.89 and 2.90), which clearly showed the surface imprinting process allowed to introduce the selectivity into the polymeric network. The relative selectivity coefficients, which designated the selectivity gained by the imprinting process have varied in the range of 2.36–58.35. Because being higher than 1.0, these results also confirmed the success of imprinting process for efficient selectivity against template ions. In the case of singular solution (in other words uncompetitive conditions), the relative selectivity coefficients were calculated as 3.28, 15.61 and 58.55 for Cd(II)/Zn(II), Cd(II)/Pb(II) and Cd(II)/Hg(II) pairs, respectively. The variation in the coefficients depended on the size and affinity for the heavy metal ions. The smaller ions [Zn(II)] could diffuse into the imprinted cavities which were fixed to size and coordination sphere of Cd(II) whereas the larger ions [Pb(II) and Hg(II)] could not sufficiently do it. In the case of triple solution (competitive condition 1), the relative selectivity coefficients slightly decreased and calculated as 2.56, and 13.56 for Cd(II)/Zn(II) and Cd(II)/Pb(II) pairs, respectively. Aforementioned, the results showed the antagonistic effect and competition between heavy metal ions.31 In the case of quadruple solutions determined a further decrease in the relative selectivity coefficient due to the addition of the fourth competitor into the mixture. As summarized in Table 2, the selectivity of the materials may differ according to the initial metal ion concentration, functional ligand utilized, adsorbent materials, size, shape and porosity of the materials. Also, Cd(II) ions are the one of the mostly studies heavy metal ions due to its close relation with health and environmental issues. As compared in this table, Cd–SIP-HMs have high selectivity under competitive and uncompetitive conditions. In the light of the results, the surface imprinting via easy hypercrosslinking of thiol groups introduced a significant recognition capability to the polymeric network even though the selectivity might slightly decrease under competitive conditions because of antagonistic effects.32,33| Solution type | Ion typea | Cd–SII-HM | NI-HM | k′ | ||||
|---|---|---|---|---|---|---|---|---|
| Q, nmol g−1 | Kd, g mL−1 | k | Q, nmol g−1 | Kd, g mL−1 | k | |||
| a Metal ion concentration: 25 ppm for all metal ions. | ||||||||
| Singular | Cd(II) | 160.2 | 19.30 | — | 40.6 | 4.62 | — | — |
| Zn(II) | 55.1 | 3.78 | 5.11 | 43.6 | 2.95 | 1.56 | 3.28 | |
| Pb(II) | 22.5 | 0.93 | 20.76 | 16.9 | 3.48 | 1.33 | 15.61 | |
| Hg(II) | 44.6 | 0.37 | 52.11 | 25.6 | 5.17 | 0.89 | 58.55 | |
| Triple | Cd(II) | 109.3 | 11.36 | — | 28.8 | 2.95 | — | — |
| Zn(II) | 25.9 | 1.77 | 6.41 | 17.4 | 1.18 | 2.50 | 2.56 | |
| Pb(II) | 13.7 | 0.52 | 21.96 | 8.6 | 1.82 | 1.62 | 13.56 | |
| Quadruple | Cd(II) | 75.4 | 8.74 | — | 22 | 2.02 | — | — |
| Zn(II) | 19.4 | 1.28 | 6.84 | 11.6 | 0.69 | 2.90 | 2.36 | |
| Pb(II) | 8.8 | 0.34 | 25.48 | 5.1 | 0.95 | 2.13 | 11.96 | |
| Hg(II) | 20.6 | 0.16 | 53.47 | 7.2 | 1.40 | 1.44 | 37.13 | |
| Polymer | Metals | Cions, ppm | The relative selectivity, k′ | Qions, mg g−1 | Interaction time, min | Reuse number | Ref. |
|---|---|---|---|---|---|---|---|
| Mesoporous silica (Cd-IMS) | Cd(II)/Pb(II) | 112.4 | 1.08 | Cd(II): 40.0 | 5 | 6 | 34 |
| Poly(HEMA–MAC) monoliths | Cd(II)/Pb(II) | 100 | 14.03 | Cd(II): 5.0 | 60 | 10 | 35 |
| Cd(II)/Zn(II) | 10.41 | ||||||
| Thiocyanato-functionalized silica gel sorbents | Cd(II)/Pb(II) | 20 | 8.9 | Cd(II): 44.7 | 20 | 6 | 36 |
| Cd(II)/Zn(II) | 11.6 | ||||||
| 2-Thiophenecarboxaldehyde-functionalized silica gel sorbents | Cd(II)/Pb(II) | 50 | 33.3 | Cd(II): 29.1 | 30 | 9 | 37 |
| Cd(II)/Zn(II) | 15.9 | ||||||
| N-Propylmaleamic acid functionalized silica gel (SG-PMA) | Cd(II)/Pb(II) | 50 | 9.46 | Cd(II): 38.3 | 20 | 6 | 38 |
| Cd(II)/Zn(II) | 11.50 | ||||||
| Amino acid-functionalized imprinted cryogels | Cd(II)/Pb(II) | 100 | 11.03 | Cd(II): 13.9 | 90 | 3 | 39 |
| Cd(II)/Zn(II) | 9.60 | Pb(II): 2.2 | |||||
| Zn(II): 2.6 | |||||||
| Magnetic poly(HEMA–MAC) beads | Cd(II)/Pb(II) | 40 | 22.6 | Cd(II): 5.5 | 60 | 10 | 40 |
| Cd(II)/Zn(II) | 160.7 | ||||||
| Organic–inorganic hybrid sorbents | Cd(II)/Pb(II) | 50 | 8.5 | Cd(II): 77.2 | 20 | 9 | 41 |
| Cd(II)/Zn(II) | 6.4 | ||||||
| Graphene oxide (GO)-based polymer | Cd(II)/Pb(II) | 10 | 3.357 | Cd(II): 83.8 | 30 | 5 | 42 |
| Cd(II)/Zn(II) | 17.278 | ||||||
| Cd(II)/Hg(II) | 4.756 | ||||||
| Amino-functionalized silica based hybrid sorbent | Cd(II)/Pb(II) | 500 | 23.3 | Cd(II): 51.6 | 20 | 9 | 43 |
| Cd(II)/Zn(II) | 14.6 | Pb(II): 15.4 | |||||
| Zn(II): 17.2 | |||||||
| Amino-functionalized silica gel sorbent | Cd(II)/Pb(II) | 50 | 3.12 | Cd(II): 57.4 | 20 | 5 | 44 |
| Cd(II)/Zn(II) | 3.00 | ||||||
| Alginic acid/acrylamide based interpenetrating polymer network | Cd(II)/Zn(II) | 5 | 6.3 | Cd(II): 48.9 | 90 | 8 | 45 |
| Imidazole incorporated sorbent | Cd(II)/Pb(II) | 2 | 157.5 | Cd(II): 4.6 | 25 | 50 | 46 |
| Cd(II)/Zn(II) | 1.38 | ||||||
| 3-Mercaptopropyltrimethoxysilane functionalized mesoporous silica | Cd(II)/Pb(II) | 202 | 8.1 | Cd(II): 61.8 | 60 | 5 | 47 |
| Surface ion imprinted hypercrosslinked monolith | Cd(II)/Pb(II) | 25 | 15.61 | Cd(II): 160.2 nmol g−1 | 45 | 10 | This study |
| Cd(II)/Zn(II) | 3.28 | Pb(II): 55.1 nmol g−1 | |||||
| Cd(II)/Hg(II) | 58.55 | Zn(II): 22.5 nmol g−1 | |||||
| Hg(II): 44.6 nmol g−1 |
3.4. Desorption and reusability
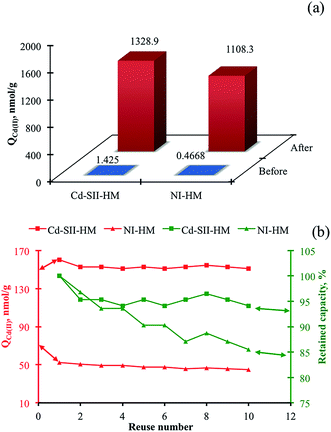
The reproducibility, repeatability, and reusability are the key features of the materials in the term of production and operational costs.32,33 In this respect, two different evaluations were performed to demonstrate the reversibility and controlled formation of imprinted cavities and to show successive reusability of the monoliths. The Cd(II) adsorption studies were carried out after denaturing the –S–S– bridges by urea solution (Fig. 7a). The straightforward increment in the adsorption capacities depended on the denaturation of the hypercrosslinks, which allowed the free thiol groups to be readily accessible for outrageous heavy metal ion adsorption capacities. Secondly, ten successive adsorption–desorption cycles were performed by using Cd–SII-HMs. After each Cd(II) ion adsorption, the Cd–SII-HMs were treated with 50 mM EDTA to desorb Cd(II) ions and then with 50 mM NaOH to regenerate the monoliths (Fig. 7b). By this desorption process, the desorption rate was achieved around 95% for each step which indicated both the efficient desorption with EDTA and the reversible nature of the interaction between heavy metal ions and functional groups. After the tenth cycle, the adsorption capacity for Cd–SII-HM decreased from 160.2 nmol g−1 to 150.8 nmol g−1 whereas that for NI-HM decreased from 52.5 nmol g−1 to 44.8 nmol g−1. The retained capacities for Cd–SII-HM and NI-HM were determined as 94.1% and 85.5%, respectively after the tenth cycle. These results showed the reproducibility of the method developed here beside the reusability of the materials, which indicated the cost-friendship of both materials and method.3.5. Mathematical modeling
Several adsorption isotherms such as Langmuir and Freundlich isotherms as well as adsorption kinetic models such as pseudo-first order- and pseudo-second order kinetics have been applied to analyze the adsorption process for last decades.31–33,39 Herein, we also utilized these mathematical operations to describe the Cd(II) ion adsorption onto surface ion imprinted (Cd–SII-HM) and non-imprinted (NI-HM) monoliths. Also, we calculated the thermodynamics parameters, i.e. Gibbs free energy (ΔG), enthalpy (ΔH), and entropy (ΔS) by using van't Hoff and Gibbs equations. All equations and description of parameters were summarized below.Adsorption isotherms
Langmuir
| 1/Qeq = (1/Ceq) (1/b Qmax) + 1/Qmax | (4) |
Freundlich
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Qeq = ln Qeq = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF + (1/n) ln KF + (1/n) ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ceq Ceq
| (5) |
Kinetic modelsp
Pseudo-first order
| log(Qeq − Qt) = log(Qeq) − (k1t)/2.303 | (6) |
Pseudo-second order
| t/Qt = (1/k2Qeq2) + (1/Qeq) t | (7) |
Thermodynamics
Van't Hoff
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b = −(ΔH/RT) + (ΔS/R) b = −(ΔH/RT) + (ΔS/R)
| (8) |
Gibss free energy
ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b b
| (9) |
Adsorption isotherms were given in ESI File (Fig. SI-9–12†). As seen in the figure, the adsorption process has a better correlation with Langmuir isotherm in accordance to the linear regression (R2) coefficient. In the light of assumptions for Langmuir isotherm, a monolayer adsorption occurred on the matrix having homogeneously distributed active sites with equal energy level. The value of 1/n for Cd–SII-HM confirmed this observation because its value is so close to 1 indicating the monolayer coverage of the surface. Also, the heavy metal adsorption on active sites (both imprinted and non-imprinted) occurred through coordinated-covalent interactions which limited the multilayer adsorption process due to the competitive affinity between the complementary species and repulsion effects between heavy metals ions in solid and liquid phases. Herein, the actual adsorption capacity for Cd–SII-HM is closely related to the theoretical values although that for NI-HM is lower than theoretical values which also indicated selective recognition ability gained by hyper-crosslinking process beside multipoint interaction between free thiol groups of NI-HM and heavy metal ions. In the light of data calculated for kinetic models (ESI File, Fig. SI-13 and 14†), pseudo-first order kinetic variables are well-fitted to heavy metal adsorption on Cd–SII-HM whereas NI-HM follows to pseudo-second order kinetics. This result depends on the creation of microporous network on the polymeric surface during hypercrosslinking process which caused a kind of diffusional limitations for Cd–SII-HM. However, the linear regression coefficient for Cd–SII-HM (0.9881) is quite high (actually higher than acceptable level >95%), which pointed out that the surface imprinting approach diminished the diffusional limitations due to the microporosity. The calculated parameters for adsorption isotherm and kinetic models were summarized in Table 3. By applying van't Hoff and Gibbs free energy equations to experimental data (ESI File, Fig. SI-15–18†), we have also calculated the thermodynamics parameters including ΔG298K, ΔH° and ΔS° values for this system as 14.02 kJ mol−1, −61.47 kJ mol−1 and −253.31 J mol−1 K, respectively. The negative signs for ΔH° and ΔS° values indicated the adsorption process is diminished by the increase in temperature and spontaneously occurs at a lower temperature. Although the positive sign of Gibbs free energy (at 25 °C) implied non-spontaneity of the process, the process is driven by the change in entropy of surroundings due to the exothermic nature of the process (negative value of enthalpy).
| Adsorption isotherms | |||||
|---|---|---|---|---|---|
| Langmuir | Freundlich | ||||
| Cd–SII-HM | NI-HM | Cd–SII-HM | NI-HM | ||
| Qmax, μmol g−1 | 1.701 | 0.289 | KF | 1.47 × 10−3 | 1.27 × 10−3 |
| b, L mg−1 | 6.2 × 10−3 | 2.4 × 10−3 | 1/n | 0.9597 | 0.6678 |
| R2 | 0.94842 | 0.80943 | R2 | 0.96290 | 0.89452 |
| Kinetics models | |||||
|---|---|---|---|---|---|
| Pseudo-1st order | Pseudo-2nd order | ||||
| Cd–SII-HM | NI-HM | Cd–SII-HM | NI-HM | ||
| Qeq, nmol g−1 | 157.5 | 50.0 | Qeq, nmol g−1 | 188.7 | 62.9 |
| k1, min−1 | 5.0 × 10−2 | 4.7 × 10−2 | k2, g nmol−1 min | 2.68 × 10−6 | 4.02 × 10−6 |
| R2 | 0.99054 | 0.96012 | R2 | 0.98810 | 0.98991 |
3.6. Practical application for real samples
In order to demonstrate the applicability of the proposed system for complex media, some extraction studies were performed Cd(II) from both tap water and synthetic seawater samples which were spiked with Cd(II) ion in two different concentration (5 and 10 ppm). In the light of Pearson acid-base classification, thiols groups naturally tend to interact with Cd(II) ions.39 For this reason, we have synthesized and used the polymerizable derivative of cysteine having thiol side chain. The Cd(II) ion concentration was compared in respect to spiked and extracted values beside recovery (%) and relative standard deviation (RSD) values (Table 4). As clearly seen in the table, the extraction performance of Cd–SII-HM is quite high for both media as well as both spiked concentration (for all cases higher than 97.5%). This result indicated the applicability of the proposed monoliths for even complex aqueous media such as synthetic seawater or tap water in addition to implying its good stability and repeatability with acceptable RSD values.4. Conclusion
The selective and cost friendly materials have been attracted a considerable attentions due to the continuing demands on the separation and purification sciences.19,20 Herein, we focused on combining the advantages of the monoliths with surface imprinting and hypercrosslinking techniques to create a synergy for developing a selective adsorbent. The selective Cd(II) ion adsorption was demonstrated not only from singular solutions (uncompetitive) but also from triple and quadruple solutions (competitive). The method developed in this study could be classified as easy-to-apply, reversible, cheap, reproducible and efficient while the materials proposed were selective, specific and reusable in the light of the results. In addition, the hypercrosslinking and surface imprinting introduced high selectivity into the polymeric network. Moreover, the monoliths showed no significant decrease in the adsorption capacity after ten successive adsorption–desorption cycle besides that using the same Cd–SII-HM column for all experiments. As a conclusion, both of method and materials are promising for the developing a novel cost-friendly and efficient adsorbents for selective and specific analyte adsorption not only from pure solutions but also complex mixtures.References
- W. W. Ngah and M. Hanafiah, Bioresour. Technol., 2008, 99, 3935–3948 CrossRef PubMed.
- F. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS PubMed.
- M. Tang, C. Xu, N. Lin, K. Liu, Y. Zhang, X. Yu and W. Liu, Chemosphere, 2016, 148, 270–275 CrossRef CAS PubMed.
- M. Gawin, J. Konefal, B. Trzewik, S. Walas, A. Tobiasz, H. Mrowiec and E. Witek, Talanta, 2010, 80, 1305–1310 CrossRef CAS PubMed.
- G.-Z. Fang, J. Tan and X.-P. Yan, Anal. Chem., 2005, 77, 1734–1739 CrossRef CAS PubMed.
- P. Joseph, Toxicol. Appl. Pharmacol., 2009, 238, 272–279 CrossRef CAS PubMed.
- A. Cuypers, M. Plusquin, T. Remans, M. Jozefczak, E. Keunen, H. Gielen, K. Opdenakker, A. R. Nair, E. Munters and T. J. Artois, BioMetals, 2010, 23, 927–940 CrossRef CAS PubMed.
- X. Luo, S. Luo, Y. Zhan, H. Shu, Y. Huang and X. Tu, J. Hazard. Mater., 2011, 192, 949–955 CrossRef CAS PubMed.
- L. Uzun and A. P. F. Turner, Biosens. Bioelectron., 2016, 76, 131–144 CrossRef CAS PubMed.
- B. Gao, F. An and Y. Zhu, Polymer, 2007, 48, 2288–2297 CrossRef CAS.
- H. Li, G. Li, Z. Li, C. Lu, Y. Li and X. Tan, Appl. Surf. Sci., 2013, 264, 644–652 CrossRef CAS.
- R. Say, M. Erdem, A. Ersöz, H. Türk and A. Denizli, Appl. Catal., A, 2005, 286, 221–225 CrossRef CAS.
- G. O. Ince, E. Armagan, H. Erdogan, F. Buyukserin, L. Uzun and G. Demirel, ACS Appl. Mater. Interfaces, 2013, 5, 6447–6452 CAS.
- Y. Saylan, R. Uzek, L. Uzun and A. Denizli, J. Biomater. Sci., Polym. Ed., 2014, 25, 881–894 CrossRef CAS PubMed.
- K. Araki, M. Goto and S. Furusaki, Anal. Chim. Acta, 2002, 469, 173–181 CrossRef CAS.
- B. Gao, Y. Li and Z. Zhang, J. Chromatogr. B, 2010, 878, 2077–2086 CrossRef CAS PubMed.
- F. X. Gao, X. T. Ma, X. W. He, W. Y. Li and Y. K. Zhang, Colloids Surf., A, 2013, 433, 191–199 CrossRef CAS.
- V. A. Davankov, C. S. Sychov, M. M. Ilyin and K. O. Sochilina, J. Chromatogr. A, 2003, 987, 67–75 CrossRef CAS PubMed.
- M. P. Tsyurupa and V. A. Davankov, React. Funct. Polym., 2002, 53, 193–203 CrossRef CAS.
- Y. Lv, Z. Lin and F. Svec, Anal. Chem., 2012, 84, 8457–8460 CrossRef CAS PubMed.
- D. Bratkowska, R. M. Marce, P. A. G. Cormack, D. C. Sherrington, F. Borrull and N. Fontanals, J. Chromatogr. A, 2010, 1217, 1575–1582 CrossRef CAS PubMed.
- K. Okada, M. Nandi, J. Maruyama, T. Oka, T. Tsujimoto, K. Kondoh and H. Uyama, Chem. Commun., 2011, 47, 7422–7424 RSC.
- K. F. Du, D. Yang and Y. Sun, J. Chromatogr. A, 2007, 1163, 212–218 CrossRef CAS PubMed.
- A. Podgornik, A. Savnik, J. Jancar and N. L. Krajnc, J. Chromatogr. A, 2014, 1333, 9–17 CrossRef CAS PubMed.
- H. Koku, R. S. Maier, K. J. Czymmek, M. R. Schure and A. M. Lenhoff, J. Chromatogr. A, 2011, 1218, 3466–3475 CrossRef CAS PubMed.
- P. Jandera, J. Chromatogr. A, 2013, 1313, 37–53 CrossRef CAS PubMed.
- A. Denizli, B. Garipcan, A. Karabakan, R. Say, S. Emir and S. Patir, Sep. Purif. Technol., 2003, 30, 3–10 CrossRef CAS.
- M. Zougagh, P. C. Rudner, A. G. de Torres and J. M. C. Pavón, J. Anal. At. Spectrom., 2000, 15, 1589–1594 RSC.
- L. Uzun, D. Türkmen, E. Yilmaz, S. Bektaş and A. Denizli, Colloids Surf., A, 2008, 330, 161–167 CrossRef CAS.
- L. Uzun, R. Say and A. Denizli, React. Funct. Polym., 2005, 64, 93–102 CrossRef CAS.
- B. Tabakli, A. A. Topçu, S. Doker and L. Uzun, Ind. Eng. Chem. Res., 2015, 54, 1816–1823 CrossRef CAS.
- L. Uzun, A. Kara, B. Osman, E. Yılmaz, N. Beşirli and A. Denizli, J. Appl. Polym. Sci., 2009, 114, 2246–2253 CrossRef CAS.
- M. M. Sari, C. Armutcu, N. Bereli, L. Uzun and A. Denizli, Colloids Surf., B, 2011, 84, 140–147 CrossRef CAS PubMed.
- W. Li, R. He, L. Tan, S. Xu, C. Kang, C. Wei and Y. Tang, J. Sol–Gel Sci. Technol., 2016, 78, 632–640 CrossRef CAS.
- S. Asir, L. Uzun, D. Turkmen, R. Say and A. Denizli, Sep. Sci. Technol., 2005, 40, 3167–3185 CrossRef CAS.
- Z. C. Li, H. T. Fan, Y. Zhang, M. X. Chen, Z. Y. Yu, X. Q. Cao and T. Sun, Chem. Eng. J., 2011, 171, 703–710 CrossRef CAS.
- H. T. Fan, J. X. Liu, H. Yao, Z. G. Zhang, F. Yan and W. X. Li, Ind. Eng. Chem. Res., 2013, 53, 369–378 CrossRef.
- M. Li, C. Feng, M. Li, Q. Zeng, Q. Gan and H. Yang, Appl. Surf. Sci., 2015, 332, 463–472 CrossRef CAS.
- M. Jalilzadeh, L. Uzun, S. Senel and A. Denizli, J. Appl. Polym. Sci., 2016, 133, 43095, DOI:10.1002/app.43095.
- N. Candan, N. Tüzmen, M. Andac, C. A. Andac, R. Say and A. Denizli, Mater. Sci. Eng., C, 2009, 29, 144–152 CrossRef CAS.
- J. B. Wu and Y. L. Yi, Korean J. Chem. Eng., 2013, 30, 1111–1118 CrossRef CAS.
- Y. Liu, X. Hu, M. Meng, Z. Liu, L. Ni, X. Meng and J. Qiu, Chem. Eng. J., 2016, 302, 609–618 CrossRef CAS.
- H. T. Fan, Y. Lu, A. J. Liu, B. Jiang, H. Shen, C. C. Huang and W. X. Li, Anal. Chim. Acta, 2015, 897, 24–33 CrossRef CAS PubMed.
- H. T. Fan, J. Li, Z. C. Li and T. Sun, Appl. Surf. Sci., 2012, 258, 3815–3822 CrossRef CAS.
- G. Parameswaran and B. Mathew, Adv. Environ. Chem., 2014, 2014, 394841 Search PubMed.
- M. G. Segatelli, V. S. Santos, A. B. T. Presotto, I. V. P. Yoshida and C. R. T. Tarley, React. Funct. Polym., 2010, 70, 325–333 CrossRef CAS.
- G. Z. Fang, J. Tan and X. P. Yan, Sep. Sci. Technol., 2005, 40, 1597–1608 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra17150h |
| This journal is © The Royal Society of Chemistry 2016 |