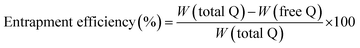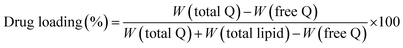Quercetin-loaded solid lipid nanoparticles improve osteoprotective activity in an ovariectomized rat model: a preventive strategy for post-menopausal osteoporosis
Naseer Ahmad†
a,
Venkatesh Teja Banala†b,
Priyanka Kushwahaa,
Anirudha Karvandea,
Shweta Sharmab,
Ashish Kumar Tripathia,
Ashwni Vermab,
Ritu Trivedi*a and
Prabhat Ranjan Mishra*b
aDivision of Endocrinology, Central Drug Research Institute (Council of Scientific and Industrial Research), Sector 10, Jankipuram Extension, Sitapur Road, Lucknow 226031, Uttar Pradesh, India. E-mail: ritu_trivedi@cdri.res.in; Fax: +91-522 2771941; Tel: +91-522 2772450
bDivision of Pharmaceutics, Central Drug Research Institute (Council of Scientific and Industrial Research), Sector 10, Jankipuram Extension, Sitapur Road, Lucknow 226031, Uttar Pradesh, India. E-mail: prabhat_mishra@cdri.res.in
First published on 21st September 2016
Abstract
A formulation of quercetin-based solid lipid nanoparticles (QSLNs) was developed to increase the bioavailability of quercetin, with an aim to evaluate its effects on bone health in comparison to free quercetin (Q). The QSLNs were prepared by emulsification solvent evaporation followed by a cold homogenization method. The QSLNs were spherical when observed under atomic force microscopy, with an average diameter of 172.9 ± 12.65 nm. This formulation was pharmaceutically characterized and then evaluated for osteoprotective activity in ovariectomized (OVx) rats. A single oral dose of QSLNs (5 mg kg−1 d−1) significantly increased the bioavailability compared to free quercetin. The oral administration of QSLNs to ovariectomized rats increased serum quercetin levels by 3.5-fold compared to free quercetin. After 12 weeks of treatment, the bone mineral density of the femur, tibia and lumbar spine L-5 measured by micro-computed tomography (μCT) was restored in the QSLNs group, and was equivalent to the sham control group compared to in the OVx group, but the QSLNs had no effect on adipogenesis and uterine weight in OVx rats. μCT analysis showed that the QSLNs group had an improved trabecular microarchitecture in the distal femoral, proximal tibial and lumbar spine cancellous bones. The developed quercetin formulation based on solid lipid nanoparticles inhibited bone loss in osteopenic rats. Q and QSLNs inhibited the receptor activator of nuclear factor-kappa B ligand (RANKL)-induced osteoclast cells differentiation and the expression of osteoclast-specific genes in in vitro experiments using bone marrow cells treated with RANKL and M-CSF. The data from this study suggest that, overall, QSLNs treatment recovers bone loss (femur, BV/TV; ∼21%, p < 0.01) more so than in the Q treatment group, while both inhibit bone loss without showing any hyperplasic effect on the uteri in OVx rats.
Introduction
Flavonoids are a group of natural polyphenols with a ubiquitous distribution in the plant kingdom as well as being widely distributed in our daily diet. Among the many flavonoids, quercetin (Q, 3,3′,4′,5,7-pentahydroxyflavone) is the major representative of its flavonol subclass. It has been shown to have a wide spectrum of biological and pharmacological activities, including dilating coronary arteries and decreasing blood lipids, as well as having anti-platelet aggregation, anti-cancer, anti-oxidation, anti-anaemia, anti-inflammation and anti-anaphylaxis effects.1 Considering the beneficial effects of the chemoprophylaxis and therapeutic effects of quercetin, there is a growing interest in its use for the treatment of osteoporosis. Recent evidence confirmed the osteoprotective activity of the dietary quercetin in in vitro and in vivo studies.2 Studies from our group have reported that quercetin has osteoprotective effects in inhibiting osteoclastogenesis and in mitigating ovariectomy-induced bone loss in rats.3 Furthermore, it can also reverse multidrug-resistance in cancer cells4,5 and enhance the anti-cancer effects of other drugs.6,7To show the in vivo activity, it must be shown to have good absorption and distribution in various body tissues. However, due to its low hydrophilicity and due to PgP efflux it has only very minimal absorption via the gastrointestinal tract, with a bioavailability of less than 17% in rats8 and 1% in humans,9 due to which the clinical application of the drug is restricted. Therefore, the need for an oral formulation with improved absorption is highly desired. Solid lipid nanoparticles, which represent a colloidal submicron particulate delivery system, have gained an increasing attention and are regarded as an effective alternative carrier system for efficient oral delivery compared to conventional emulsions and polymeric microparticles.10,11
WHO- and FDA-approved edible solid lipids have been used as carriers, where the drug molecules were encapsulated to obtain the solid lipid nanoparticles.12–14 The advantages of the system include high biocompatibility, high bioavailability, controlled release, and no problems with multiple routes of administration, such as oral, intravenous, pulmonary, and transdermal administration.15–20 Sufficient data implicate that the bioavailability of poorly hydrophilic and lipophilic drugs can be improved when these drugs are encapsulated in SLNs.16,21 There are numerous reports explaining the oral bioavailability enhancement of flavonoids via SLNs, Vandita et al. claimed a 39-fold enhancement of the oral bioavailability of curcumin compared to curcumin solution in vivo.22 Similarly Houli et al. reported a 571.4% enhancement in the relative bioavailability of quercetin with a significant increase in pharmacokinetic parameters.23 Souto et al. developed SLNs of cyclosporine, in which the authors demonstrated the advantages of SLNs over nanocrystal formulations with a comparable enhancement of the bioavailability.24 Nitrendipine-loaded SLNs were reported by Venishetty et al., involving an antihypertensive drug, which was lipophilic with poor oral bioavailability ranging from 10% to 20% but had shown a 3–4-fold increment in bioavailability compared to its suspension formulation.25
The aim of the present study was to design quercetin-solid lipid nanoparticles (QSLNs) and then to assess the bioavailability of these QSLNs, as well as to evaluate the potential of quercetin in an ovariectomized rat model of osteoporosis using SLNs as an oral delivery carrier. To formulate the SLNs, emulsion solvent evaporation followed by a cold homogenization method was used.26–29
Postmenopausal osteoporosis results in the increased resorption of bone, which, in turn, leads to increased bone fragility and fractures.30 Being phytoestrogens, flavonoids have gained a preference as prophylactic agents against postmenopausal bone loss because of their safety profile and lack of adverse effects that often occur with hormone therapy.31–36 Quercetin (Q), being a major dietary flavonoid, has recently been shown to reduce bone loss in ovariectomized (OVx) mice.2 Some evidence has demonstrated the inhibition of osteoclast differentiation and the expression of osteoclastogenic marker genes upon quercetin exposure in an osteoclast precursor cell line, RAW 264.7 (mouse monocyte/macrophage) cells.37–39 Q does not have a uterine oestrogenic response and fails to activate oestrogen receptors in the reporter assay system, thereby suggesting its possible therapeutic use in postmenopausal osteoporosis.2,40
The intake of flavonol-rich diets (a subclass of flavonoids that includes kaempferol and quercetin) has been positively associated with better skeletal health in humans.3,36 In animal models, the counteraction or reversal of bone deleterious effects and oestrogen deficiency have been demonstrated with flavonol-rich dietary supplements and nutraceuticals.36 Furthermore, treating osteopenic rats with quercetin mitigates bone loss without having a uterine hyperplastic effect.3 Quercetin imparts a bone sparing action induced by its anti-oxidant properties that assuage the production of oxidation-derived free radicals from the bone-resorbing osteoclasts and their precursors.41,42 Despite its so many positive attributes, quercetin has poor bioavailability, which impedes the application of this promising and relatively non-toxic agent to develop it as a pharmacological agent for postmenopausal osteoporosis. We hypothesize that overcoming the bioavailability issues of quercetin could improve osteoprotective activity in osteopenic rats. In the present study, we focused on developing solid lipid nanoparticles bearing quercetin (QSLNs) as a two-stage approach, first, to improve its oral bioavailability and second to assess its enhanced restorative effect in an osteoporotic animal model in long-term treatment involving associated cellular mechanisms.
Interestingly, it has been suggested that onion bulbs may be associated with an increase in BMD.43 Horcajada-Molteni et al.44 previously reported that rutin inhibits trabecular bone loss but has no effects on uterine weight in OVX rats. Rassi et al.45 and Woo et al.38 reported that quercetin inhibited osteoclast formation in porcine and mouse bone marrow cells and in monocytic RAW264.7 cells. However, the role of quercetin-solid lipid nanoparticles on bone loss in Ovx rats has not yet been reported. In this study, we demonstrate for the first time the effects of quercetin-solid lipid nanoparticles on bone loss by ovariectomy in vivo, and also examine the effects of quercetin-solid lipid nanoparticles.
Materials and methods
Materials
Cell culture media and supplements were purchased from Invitrogen (Carlsbad, CA). All the fine chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA). CTx (fragments of type I collagen) enzyme-linked immunosorbent assay kit was purchased from Qayee Bio-Technology Co. Ltd. (Shanghai, China). All the chemicals used in this study were of analytical grade. Quercetin and E2 were purchased from Sigma-Aldrich (St. Louis, MO, USA).Preparation of quercetin-solid lipid nanoparticles (QSLNs)
QSLNs were prepared by emulsion solvent evaporation followed by a high pressure homogenization (HPH) technique, with the process parameters, such as pressure, number of cycles and concentration of surfactants, optimized with the objective to obtain an average size below 200 nm (data not shown). The desired amount of quercetin (Q) [150 mg] was dissolved in hot ethanol and added to molten lipids (glycerol mono stearate [300 mg], hydrogenated soya PC [100 mg] and soya PC [1000 mg]) at 70 °C and the solvent was allowed to evaporate for 2 h. Water-containing surfactant (2% Tween 80) was mixed separately and heated to the same temperature as the lipid dispersion. A hot pre-emulsion was produced by dispersing the molten lipid phase into 50 mL of the hot aqueous phase using Ultra Turrax® for 5 min at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. The hot pre-emulsion was further processed by a high pressure homogenizer (Microfluidics) at 1200 bar at 10 °C for 15 cycles to obtain the final particle size of QSLNs.46,47
000 rpm. The hot pre-emulsion was further processed by a high pressure homogenizer (Microfluidics) at 1200 bar at 10 °C for 15 cycles to obtain the final particle size of QSLNs.46,47
Characterization of QSLNs
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture) and the drug content was estimated after proper dilutions using HPLC.
1 mixture) and the drug content was estimated after proper dilutions using HPLC.The drug loading and entrapment efficiency was determined by using the following equations:
Similarly, to check the stability of the QSLNs upon oral administration, in vitro stability studies were performed in blank SGF pH 1.2 and blank SIF pH 6.8. 100 μL of QSLNs were incubated in 2 mL of SGF/SIF in a thermostatic shaker bath maintained at 37 °C and 100 rpm. The samples were withdrawn at serial different time intervals of 0, 15, 30, 60, 90 and 120 min. After incubation for specified time periods, the samples were analysed for their change in size and PDI using the Malvern Zetasizer Nano ZS, as reported above.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetonitrile (1
acetonitrile (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added. The resulting mixtures were vortexed vigorously for 4 min and the supernatant was collected in separate test tubes after centrifuging the samples at 10
1) was added. The resulting mixtures were vortexed vigorously for 4 min and the supernatant was collected in separate test tubes after centrifuging the samples at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min. The procedure was repeated twice and the collected supernatant was dried with the help of a Maxi dryer (Gemini BV Laboratory, Netherlands). The dried samples were reconstituted with 100 μL of the mobile phase, and 40 μL was injected into a Shimadzu HPLC system equipped with a Waters RP-18 column and UV detector at a wavelength of 370 nm with a mobile phase of acetonitrile and 1% acetic acid, (22
000 rpm for 15 min. The procedure was repeated twice and the collected supernatant was dried with the help of a Maxi dryer (Gemini BV Laboratory, Netherlands). The dried samples were reconstituted with 100 μL of the mobile phase, and 40 μL was injected into a Shimadzu HPLC system equipped with a Waters RP-18 column and UV detector at a wavelength of 370 nm with a mobile phase of acetonitrile and 1% acetic acid, (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78% v/v) (pH 3.04) at a flow rate of 1.0 mL min−1.
78% v/v) (pH 3.04) at a flow rate of 1.0 mL min−1.Experimental design
Adult female Sprague Dawley rats (∼180–200 g) were taken for the study and were bilaterally ovariectomized (Ovx) in various treatment groups, while rats of the control group were exposed to a sham surgical procedure. All the rats were individually housed at 21 °C, in 12 h/12 h light-dark cycles. A normal chow diet and water were provided ad libitum. Treatment started 24 h after the surgery and continued for 12 weeks. Both quercetin (Q) and quercetin-solid lipid nanoparticles (QSLNs) at 5.0 mg per kg per day treatment by oral gavage was given.3 Each group consisted of 10 rats, and the groups were as follows: sham operated (ovary intact) + vehicle (gum acacia), OVx + vehicle, OVx + 5.0 mg per kg per day Q, OVx + 5.0 mg per kg per day QSLNs and OVx + E2 (2.5 μg per kg per day). At the end of 12 weeks of oral gavage treatment, all the rats were first acclimatized and then kept in metabolic cages with no food but only water for 24 h to collect urine samples to measure the urinary fragments of type I collagen (CTx) as a marker of bone resorption using an ELISA kit, and then the rats were euthanized with ketamine and xylazine. At autopsy, the uteri were collected for uterine histomorphometry analysis and the uterine weight measured to verify that the ovaries were removed successfully. Bones were dissected and separated from the adjacent tissue, cleaned, and bone marrow extracted for the ex vivo studies, followed by storage of the femur and tibia at −80 °C for RNA isolation. The femur, tibia and vertebrae were used for micro-architectural determination.Measurement of bone mineral density (BMD)
Volumetric BMD analysis of the femur, tibia and vertebrae was performed from the VOI made for the trabecular region by using μCT scans. Calibration was done using hydroxyapatite (HA) phantom rods of 2 mm diameter with known BMD (0.25 g cm−3 and 0.75 g cm−3) following the previously published protocols.48,49 Analysis was performed based on a linear correlation between the micro-CT attenuation coefficient and bone mineral density.48,49Micro-computed tomography (μCT)
The QSLNs effects on the trabecular part of the metaphysis region in OVx-induced bone deterioration were evaluated with μCT (SkyScan 1076; Aartselaar, Belgium) following a previously published protocol with little modification.50 Long bones (the femora and tibiae) and vertebrae were dissected out during the autopsy, and the soft tissue was cleaned off and fixed in 4.0% formaldehyde before storage in alcohol. Scanning of the bone samples was performed at a nominal resolution (pixels) of 18 μm using the SkyScan software. Reconstruction of the images was carried out using the modified Feldkamp algorithm, which was facilitated by a network-distributed reconstruction performed on four personal computers running simultaneously. The X-ray source was set at 70 kV and 100 mA, with a pixel size of 18 μm. A hundred projections were obtained at an angular range of 180°. Reconstruction of the image slices was performed by using cone beam reconstruction software, version 2.6, based on the Feldkamp algorithm (SkyScan). Trabecular bone extraction was done by drawing ellipsoid contours with the help of CT analyzer software. The trabecular bone volume (BV/TV, %), trabecular number (Tb N, mm−1) and trabecular separation (Tb Sp, mm) were calculated by using the mean intercept length method. The trabecular thickness (Tb Th, mm) was calculated according to the method of Hildebrand and Rüegsegger.51 The structure model index (SMI, an indicator of plates and rods relative to their prevalence in bone) was acquired via the method of Hildebrand and Rüegsegger.51 Calculation of the degree of anisotropy (DA) was done by the mean intercept method, which describes the magnitude of the preferred orientation of the trabeculae.52 An index known as the ‘connectivity’ (Conn) was obtained to illustrate the redundancy of the trabecular connections. The connectivity was derived from the Euler number,53 which is a fundamental topologic measure that counts the number of objects, the number of marrow cavities surrounded by bone and the number of connections that must be broken to divide the structure into two parts. Since the connectivity relies on the structure size, it is more appropriate to describe this index as a density [connectivity density (Conn Dn)] by dividing it by the total volume.53 The three-dimensional (3D) parameters were based on an analysis of a marching cubes-type model with a rendered surface.Bone strength
The femora were subjected to three-point bending, and the fifth lumber vertebrae were subjected to compressive strength using a bone strength tester (model TK-252C; Muromachi Kikai, Co. Ltd., Tokyo Japan) following our previously published protocol.54–56Induction of adipocyte differentiation in bone marrow cells (BMCs)
For adipogenic differentiation, 1 × 107 BMCs were seeded in 24-well plates and cultured in an adipogenic medium [Dulbecco's modified Eagle's medium (DMEM)] containing 1.0 μM dexamethasone, 0.5 mM isobutylmethylxanthine (IBMX), 100 μM indomethacin, 10% FBS and insulin (10 μg mL−1) for 7 days. On day 3, the medium was replaced with complete growth medium containing only insulin (10 μg mL−1). Cultures of bone marrow cells from different treated groups were performed for 21 days towards adipogenic differentiation and the medium was changed on every third day. The cells were fixed after 21 days in 4% paraformaldehyde and stained with Oil Red O. To quantify the incorporation of the lipid, the area stained with Oil Red O was measured by taking photomicrographs.57–59 500 μL of 70% isopropanol was added to the stained cells for the extraction of the Oil Red O stain, and these were then left at 37 °C for 20 min.57 The extracted stain from each group was quantified using an ELISA plate reader at 490 nm.Osteoclasts culture and differentiation from the bone marrow precursor cells
A primary cell culture of osteoclast was performed following the previously published protocol.60 Bone marrow macrophage cells were isolated from the marrow of 4 to 6 week-old BALB/c mice and cultured overnight in α-minimal essential medium with penicillin and streptomycin supplemented with 10% fetal bovine serum and 10 ng mL−1 macrophage colony-stimulating factor (M-CSF).3 The non-adherent cells were collected and centrifuged. Then, the collected cells were suspended in medium and cultured in 48-well plates in α-minimal essential medium with 10% fetal bovine serum with M-CSF (10 ng mL−1) and the receptor activator for nuclear factor κB ligand (RANKL; 50 ng mL−1).3TRAP staining for osteoclasts
Tartrate-resistant acid phosphatase (TRAP) staining was performed according to the previously published protocol.60 Cells were cultured and treated with a 20 μM concentration of QSLNs and Q.3 The medium and supplements (M-CSF and RANKL) were refreshed once every 2 days. The culture was continued for a total of 7 days. Then, the cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP) activity. Multinucleated (≥3 nuclei) TRAP-positive cells were counted as osteoclasts under microscopic examination.3RNA analysis by real-time PCR
qRT-PCR analysis was performed to measure the expression of osteoclast-specific genes from osteoclast cells cultured from bone marrow cells treated with RANKL + M-CSF (control), Q (20 μM) + RANKL + M-CSF and QSLNs (20 μM) + RANKL + M-CSF for a total period of 7 days following the previously published protocol.50,60 Total RNA was isolated from osteoclast cells cultured from bone marrow cells treated with Q and QSLNs and from frozen long bones (devoid of bone marrow) of rats from various groups. The bones were pulverized under liquid nitrogen and further homogenized in TRIzol reagent (Invitrogen Life technologies, USA). The concentration and purity of the RNA samples were determined by measuring the absorbance at 260 nm and the ratio at 260 nm and 280 nm (A260/A280), respectively. Primers were designed using the Universal Probe Library (Roche Applied Sciences), while cDNA was synthesized with the Revert Aid cDNA synthesis kit (Fermentas, Austin, USA) using 2.0 μg of total RNA. SYBR green chemistry was performed to quantitate the relative expression of transcripts of the genes. All the genes were analyzed using the Light Cycler 480 (Roche Molecular Biochemicals Indianapolis, IN, USA) real-time PCR machine, as we have reported earlier.44 Table 1 shows the primer sequences of the various genes used for the qPCR experiments.| Gene name | Primer sequence | Accession number |
|---|---|---|
| Rat genes | ||
| TRAP | F-TCTGTGGTTCCCCCTTCC | XM_006242694.2 |
| R-CCACCAGCAGCTTTTTCTCT | ||
| RANK | F: CAGCCACCACTACCACAGAG | NM_001271235.1 |
| R: CACTCATAAAACCACCCAAGG | ||
| C-fos | F-ACATCTCCGGAAGAGGTGAG | NM_022197.2 |
| R-ACCTCAAGGACTTGAAAGCATC | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Mouse genes | ||
| TRAP | F-CGTCTCTGCACAGATTGCAT | NM_001102405.1 |
| R-AAGCGCAAACGGTAGTAAGG | ||
| RANK | F-AGAGGCATTATGAGCATCTCG | BC019185.1 |
| R-CGAGTGCACTTAGAGGACAGGT | ||
Bone turnover markers
The bone turnover marker was determined on the basis of our previously published protocol.61 The assessment of urine samples from individual rats was performed for the bone turnover marker. Urinary fragments of type I collagen (CTx) levels were estimated using enzyme-linked immunosorbent assay kits (Qayee Bio-Technology Co. Ltd., Shanghai, China) by following the manufacturer's protocols.Uterine histomorphometry
At autopsy, the uteri were dissected out from all the groups of rats and gently blotted on filter paper, then weighed. For histological analysis, all the uteri were fixed in 4% paraformaldehyde. Segments of uteri of the different treated groups were dehydrated in ascending grades of isopropanol, cleared in xylene and then embedded in paraffin wax, following the previously published protocol.56,62 Transverse sections 5.0 μm in size were cut from the uteri of the various treated groups. These sections were stained with haematoxylin and eosin for representative photomicrographs using a Leica DC 300 camera fitted with a Leica DMLB microscope and Leica IM50 Image Acquisition software.56,62 Parameters for uterine histomorphometry analysis, such as the total uterine area, the luminal area and luminal epithelial height were determined using Leica Qwin software (Leica Microsystems, Bannockburn, IL, USA).62,63Statistical analysis
All the results obtained from this study are expressed as the mean ± SEM. The data obtained in the experiments with multiple treatments were analysed using one-way ANOVA followed by the Newman–Keuls test of significance with the help of Graph Pad Prism version 5.0 software. Qualitative observations were represented following assessments made by three individuals blinded to the experimental designs. Probability values of p < 0.05 were considered to be statistically significant.Results
Preparation and characterization of the QSLNs
Sufficient drug (Q) solubility in lipids is the major criterion for the preparation of SLNs. Flavonoids are compounds with a low solubility in both polar and non-polar solvents and have a tendency to reside at the interface.64 Due to their tendency to get adsorbed on the surface of oil droplets, there is a high chance of the drug leaching out of the lipid matrix. This makes the development of stable solid lipid nanoparticles difficult. In the present study, GMS, HSPC and Soya PC were used in combination. Prior to the production of the SLNs, lipid screening was performed by increasing the amount of Q in various molten lipids and then checking for the presence/or absence of crystals. Soya PC was found to be a very suitable lipid, which also had good surfactant capability. A drug-to-lipid ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 was fixed with a total lipid content of 3% w/v. A high pressure homogenizer was used for the manufacturing of the QSLNs, in which the process parameters, such as the pressure and number of cycles, were optimized to obtain a particle size less than 200 nm. Different batches were optimized in which particles of the desired size were obtained at 1200 bar pressure in 15 cycles.
10 was fixed with a total lipid content of 3% w/v. A high pressure homogenizer was used for the manufacturing of the QSLNs, in which the process parameters, such as the pressure and number of cycles, were optimized to obtain a particle size less than 200 nm. Different batches were optimized in which particles of the desired size were obtained at 1200 bar pressure in 15 cycles.
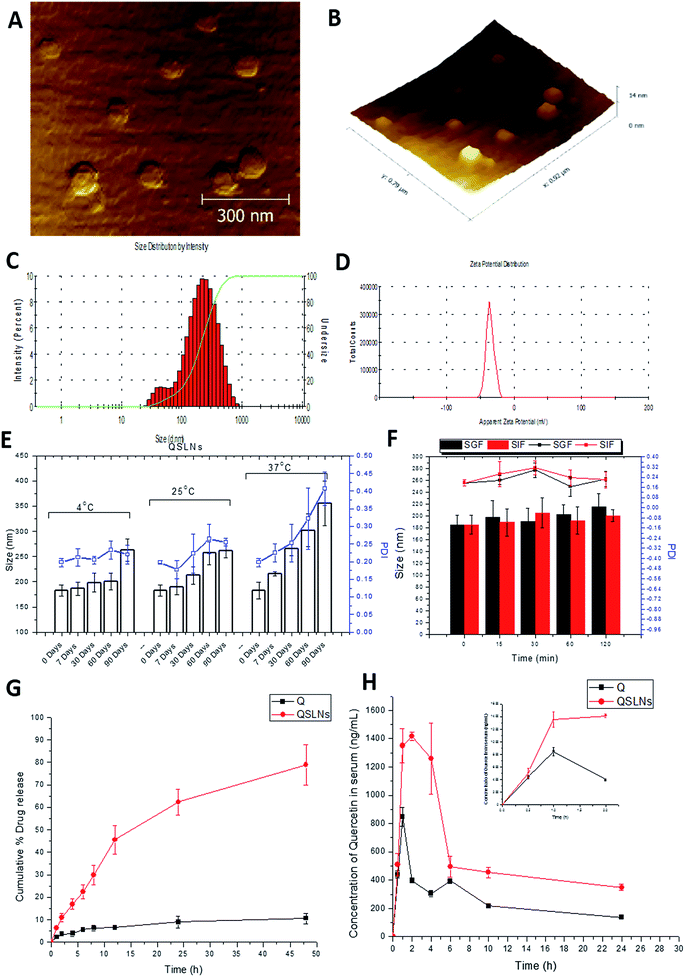
From the size measurements, made using the DLS technique, it was evident that the average mean particle size was 172.9 ± 12.65 nm with a PDI of 0.294 ± 0.056 (Fig. 1C). Similarly, the zeta potential of the particles was found to be −35.8 ± 5.80 mV (Fig. 1D), which is the stability representative of the QSLNs. The surface architecture of the QSLNs was analyzed using atomic force microscopy, and from the topological observations, it was evident that the QSLNs were spherical in shape with smooth surface with a particle size less than the observed particle size obtained from the DLS measurements (Fig. 1A and B). This may explain the discrepancy between the two analytical techniques where AFM clearly gives more authenticated results than DLS, in which the hydrodynamic diameter is calculated.56
Entrapment efficiency and drug loading
The encapsulation efficiency of the QSLNs was determined by an ultrafiltration technique, and an average entrapment efficiency of 84.33 ± 3.6% was attained, with a drug loading of 8.36 ± 0.62% and a total drug content of the formulation of 94.5%.Storage stability and in vitro stability of the QSLNs in simulated gastric/intestinal fluids
The stability of the QSLNs was analyzed at different temperature conditions (4 ± 2 °C, 25 ± 2 °C and 37 ± 2 °C) for a duration of 3 months. The size and PDI of the formulations were determined at predetermined intervals and are shown in Fig. 1E. The assessment of the change in particle size and of the PDI of the QSLNs showed they had sufficient stability over a period of 3 months. The QSLNs stored at 37 °C showed a slight increase in PDI compared to the formulation stored at 4 °C and 25 °C. The poor stability of the formulation may be due to destabilization in terms of aggregation and leaching out of the quercetin from the formulation.For oral delivery of nanoparticulate systems, gastrointestinal stability is very important. Therefore, to check the stability of the prepared QSLNs, we evaluated them in simulated gastric and intestinal fluid. After incubation for 2 h, we found the developed QSLNs to be stable with no major difference in the size or in the PDI (Fig. 1F).
In vitro drug release studies
The in vitro release study of the QSLNs was evaluated in SGF (pH 1.2) for 2 h and SIF (pH 6.8) for the remaining period of time. As shown in Fig. 1G, there was a marked difference in the release pattern of QSLNs compared to quercetin. In the case of the QSLNs, a sustained release was observed and for which around 45 ± 6.33% of the drug was released at the end of 12 h, whereas the pure quercetin release was below 10%.Effect of the solid lipid nanoparticles (SLNs) on the bioavailability of quercetin
The average serum concentrations time profiles of the QSLNs following oral administration are illustrated in Fig. 1H. The pharmacokinetic parameters were calculated using one compartmental model, as shown in Table 2. Compared to quercetin, a significant improvement in the pharmacokinetics of the QSLNs was observed. Based on the AUC0−α values, quercetin showed a significantly lower exposure than the QSLNs, whereby the QSLNs led to a 3.5-fold and 1.6-fold increase in AUC0−α and Cmax. Furthermore, the Tmax of the QSLNs was observed at 2 h, whereas for quercetin, it was observed at 1 h. The t1/2 of the formulation was increased to 46.66 h, which may be due to enhanced tissue distribution.| Q | QSLNs | |
|---|---|---|
| Cmax (ng mL−1) | 848.63 | 1415.20 |
| Tmax (h) | 1 | 2 |
| AUC0−α (ng mL−1) h | 8951 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 842.5 842.5 |
| MRT (h) | 19.94 | 42.23 |
| CL (mL h−1 kg−1) | 2792.71 | 785.11 |
| t1/2 (h) | 14.28 | 46.66 |
Effect of the quercetin-solid lipid nanoparticles (QSLNs) on BMD
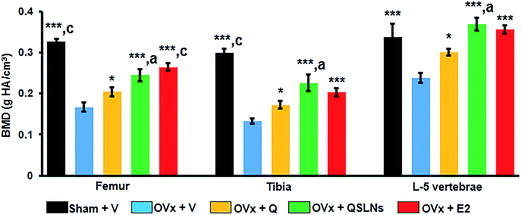
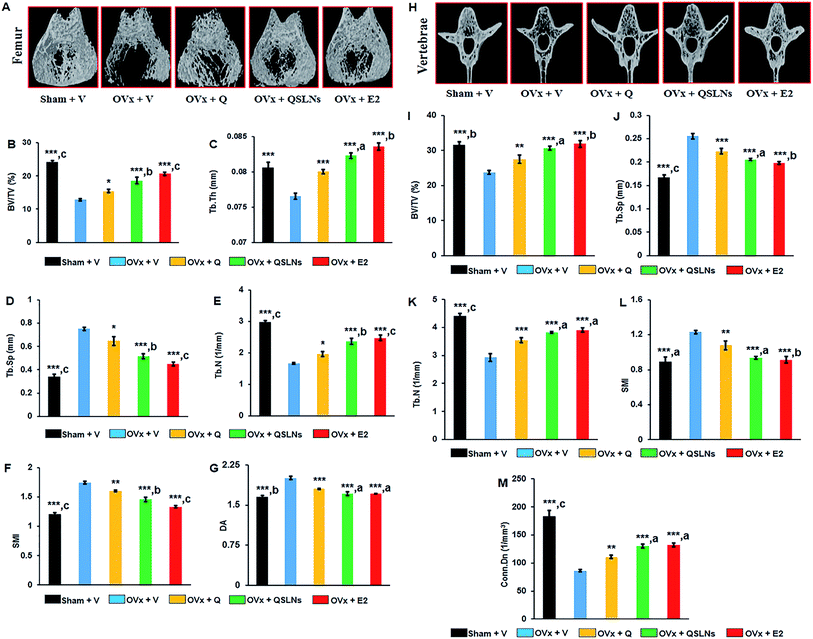
The BMD measurement results at the trabecular sites of the femur, tibia and L-5 vertebra from the different groups are shown in Fig. 2. μCT analysis was done on excised bones of all the groups to compare the BMD values. When the BMD measurements of the OVx group for distal femur metaphysis, proximal tibia metaphysis and L-5 vertebra were compared with the results from the sham group, a significant decrease by ∼49%, ∼55% and ∼30% could be observed in the OVx group, respectively (p < 0.001). The QSLNs and E2 groups showed significantly higher BMD values at the trabecular sites by ∼47% and ∼59% (femur), by ∼70% and ∼53% (tibia) and by ∼54% and ∼49% (L-5), respectively, compared to the OVx group (p < 0.001). However, on the other hand, the Q group maintained the gain in BMD values achieved during the 12 weeks of treatment to a certain extent, to such a degree that it had significantly higher BMD values at the trabecular sites of the femur, tibia and L-5 by ∼22%, ∼29%, and ∼26%, respectively, when compared to the OVx group (p < 0.05). Moreover, the BMD values at the trabecular sites of the femur, tibia and L-5 were comparable between the E2 and QSLNs groups.Effect of the quercetin-solid lipid nanoparticles (QSLNs) on the trabecular microarchitecture
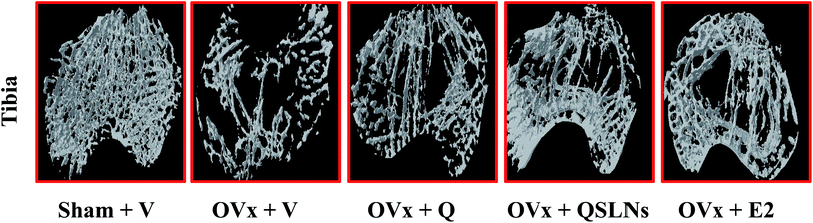
In a gross observation by 3D-μCT, trabecular bone microarchitecture deterioration due to the loss of trabecular bone of the femur and tibia was observed in the OVx group compared with the trabeculae of the femur and tibia in the sham group (representative 3D-μCT images are given in Fig. 3A and the results listed in Table 3). The trabecular response with Q or QSLNs treatment in the OVx group was assessed at the distal femoral and proximal tibial metaphysis, and the values were compared with those of the E2 group. The femoral data show that the OVx group had reduced bone volume/tissue volume (BV/TV) by ∼47% (p < 0.001, Fig. 3B), trabecular thickness (Tb Th) by ∼5% (p < 0.001, Fig. 3C) and trabecular number (Tb N) by ∼44% (p < 0.001, Fig. 3E), and increased trabecular separation (Tb Sp) by ∼122% (p < 0.001, Fig. 3D), structure model index (SMI) by ∼45% (p < 0.001, Fig. 3F) and degree of anisotropy (DA) by ∼21% (p < 0.001, Fig. 3G) when compared with the sham group. Similar to femur metaphysis, quantitative data of the trabecular regions at the proximal tibial sites for the OVx group were validated with the femur microarchitecture data and showed a decrease in BV/TV by ∼67% (p < 0.001), Tb Th by ∼15% (p < 0.001) and connectivity density (Conn Dn) by ∼76% (p < 0.001) over the sham group (Table 3). The femoral and tibial data show that the OVx group rats treated with either QSLNs or E2 reversed the OVx-induced micro-architectural changes observed in the above parameters studied, and the effects were better than those obtained for the Q-treated group.| Sham + V | OVx + V | OVx + Q | OVx + QSLNs | OVx + E2 | |
|---|---|---|---|---|---|
| a QSLNs treatment prevents tibial trabecular microarchitecture deterioration in OVx rats. Representative μCT images of tibia metaphysis of various experimental groups (upper panel of Table). Quantification of μCT data on various trabecular parameters are presented in the lower panel of Table, percentage of bone volume/tissue volume (% BV/TV), trabecular thickness (Tb Th), connection density (Conn Dn) respectively. Values are expressed as the mean ± SEM; n = 10 rats per group. *p < 0.05, **p < 0.01 and ***p < 0.001 compared with the OVx + vehicle group. ap<0.05, bp<0.01 and cp<0.001 when compared with the Q group. | |||||
| BV/TV (%) | 22.78 ± 0.63***,c | 7.38 ± 0.43 | 10.29 ± 0.65* | 13.00 ± 0.55***,a | 13.69 ± 0.66***,a |
| Tb Th (mm) | 0.084 ± 0.001***,c | 0.0714 ± 0.0003 | 0.0737 ± 0.0003* | 0.0768 ± 0.0003***,a | 0.0777 ± 0.0008***,b |
| Conn Dn (mm−3) | 82.35 ± 3.05***,c | 19.75 ± 1.32 | 27.35 ± 2.42* | 35.12 ± 2.13***,a | 42.61 ± 1.09***,c |
When the trabecular parameters were assessed in the L-5 vertebra, the OVx group had significantly reduced BV/TV by ∼25% (p < 0.001, Fig. 3I), Tb N by ∼34% (p < 0.001, Fig. 3K) and Conn Dn by ∼53% (p < 0.001, Fig. 3M) and increased Tb Sp by ∼53% (p < 0.001, Fig. 3J), and SMI by ∼38% (p < 0.001, Fig. 3L) when compared to the sham group. The OVx group treated with either Q or QSLNs reversed the OVx-induced changes observed in the above studied parameters, with the effects of QSLNs treatment being better than for the Q-treated group. Bone mass and structure deterioration in the tibia metaphysis after OVx was greater than for femur metaphysis.65 When all the parameters were considered, the osteoprotective effect with respect to the femur revealed a significantly better micro-architectural presentation than for the tibia and vertebra (L-5) in the QSLNs treatment group over the Q group.
Effect of quercetin-solid lipid nanoparticles (QSLNs) on biomechanical strength
Femoral strength and L-5 vertebral compressive strength were measured to check bone biomechanical strength by using a three-point bending test. Energy and stiffness, as indices of biomechanical strength, were reduced significantly in the OVx group by ∼53% and ∼48% (p < 0.001, femur) and by ∼21% and ∼57% (p < 0.001, L-5 vertebra) compared with the sham group (Table 4). Q and QSLNs treatment at a dose of 5 mg per kg per day to OVx rats showed a significant increase in the biomechanical strength indices in terms of the energy and stiffness in the femoral mid-shaft by ∼26%, ∼24% (Q, p < 0.05) and ∼55%, ∼53% (QSLNs, p < 0.001) and in the L-5 vertebrae by ∼11%, ∼28% (Q, p < 0.05) and ∼21%, ∼65% (QSLNs, p < 0.001), respectively, compared with the OVx group. Moreover, it was found that these two parameters in the femoral mid-shaft and L-5 vertebrae were comparable in the QSLNs and E2 groups. Together, the data from this study indicate that QSLNs prevent the loss of bone biomechanical strength of the femur and L-5 vertebrae and maintain bone quality under oestrogen deficiency.| Sham + V | OVx + V | OVx + Q | OVx + QSLNs | OVx + E2 | |
|---|---|---|---|---|---|
| a QSLNs treatment improves bone biomechanical strength in OVx rats. QSLNs-treated group shows improved femoral and vertebral strength represented by energy and stiffness, respectively. Values are expressed as the mean ± SEM; n = 10 rats per group. *p < 0.05, **p < 0.01 and ***p < 0.001 compared with the OVx + vehicle group. ap<0.05, bp<0.01 and cp<0.001 when compared with the Q group. | |||||
| Femoral bone strength | |||||
| Energy (mJ) | 71.41 ± 5.35***,c | 36.16 ± 3.85 | 45.42 ± 3.63* | 56.01 ± 5.06***,b | 57.58 ± 4.07***,b |
| Stiffness (N mm−1) | 306.21 ± 14.34***,c | 160.81 ± 20.12 | 198.93 ± 21.34* | 245.77 ± 12.77***,b | 250.61 ± 14.45***,b |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| L-5 vertebral compressive strength | |||||
| Energy (mJ) | 1040.97 ± 57.63***,b | 817.52 ± 30.51 | 906.58 ± 25.80* | 987.86 ± 40.16***,a | 1007.65 ± 56.89***,a |
| Stiffness (N mm−1) | 190.97 ± 15.83***,c | 82.35 ± 8.00 | 105.21 ± 8.00* | 135.86 ± 11.61***,b | 168.17 ± 12.58***,c |
Effect of quercetin-solid lipid nanoparticles (QSLNs) on the expression of osteoclastogenic genes
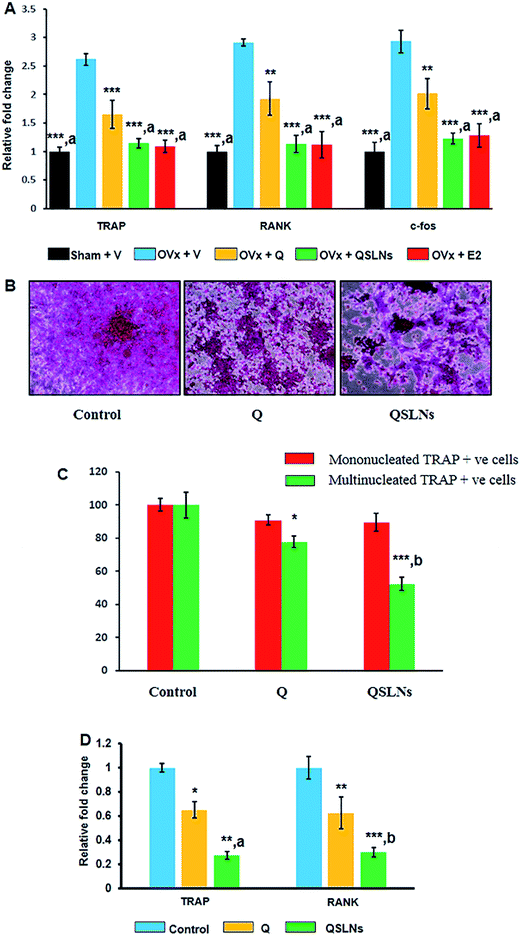
RANKL is secreted by osteoblasts and stimulates osteoclastogenesis, with an accompanying increase in the expression of osteoclast differentiation genes, such as RANK and TRAP.51 The mRNA expressions of osteoclastogenic genes, such as C-fos, TRAP and RANK, were significantly higher by ∼193% (p < 0.001), ∼162% (p < 0.001) and ∼191% (p < 0.001) in the OVx group bone (femur devoid of bone marrow) compared to in the sham group (Fig. 4A). Q, QSLNs and E2 treatment resulted in lower mRNA levels of C-fos by ∼31% (p < 0.01), ∼58% (p < 0.001) and ∼56% (p < 0.001), TRAP by ∼37% (p < 0.001), ∼56% (p < 0.001) and ∼58% (p < 0.001) and RANK by ∼34% (p < 0.01), ∼61% (p < 0.001) and ∼62% (p < 0.001), respectively, compared to the OVx group (Fig. 4A). The QSLNs-treated group exhibited significantly lower C-fos (∼39%; p < 0.05), TRAP (∼41%; p < 0.05) and RANK (∼30%; p < 0.05) mRNA levels when compared to the Q group.Fig. 4B shows the representative images of osteoclast cells by TRAP staining. Here, it was found that Q and QSLNs at 20 μM (ref. 3) concentration inhibited the formation of osteoclast cells from BMCs induced by RANKL and M-CSF, as evident by the lower multinucleated and TRAP-positive cells (Fig. 4C). The mRNA expressions of osteoclastogenic genes, such as TRAP and RANK, were significantly reduced by ∼35% (p < 0.05) and ∼37% (p < 0.01) in the Q-treated group and by ∼73% (p < 0.01) and ∼70% (p < 0.001) in the QSLNs-treated group compared with the control group (Fig. 4D). However, QSLNs at the same dose, on the other hand, significantly inhibited osteoclastogenesis (TRAP; ∼58%, P < 0.05, RANK; ∼52%, p < 0.01) from BMCs compared to Q (Fig. 4D). Together, the data from this study suggest that QSLNs are more potent in inhibiting osteoclastogenesis from bone marrow cells than Q.
Effect of quercetin-solid lipid nanoparticles (QSLNs) on the adipocyte differentiation of bone marrow cells (BMCs)
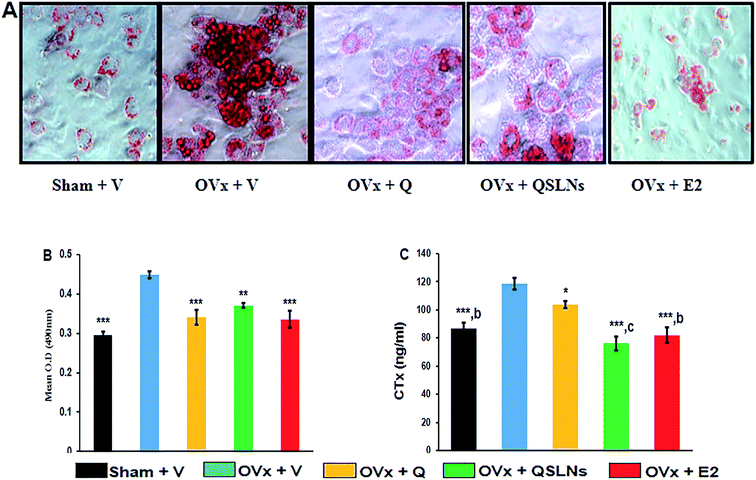
Bone marrow cells were cultured and differentiated into adipocytes, followed by Oil Red O staining, to assess the accumulation of intracellular lipid droplets. The upper panel in Fig. 5A shows representative photomicrographs of Oil Red O stained cells cultured for the differentiation of BMCs to adipocytes, while the lower panel (Fig. 5B) shows the quantitation of lipid droplets accumulation in cells by extraction of the dye from the different treatment groups. In comparison to the OVx group, Q treatment significantly reduced lipid accumulation by ∼24% (p < 0.001) and led to fewer lipid filled cells as did QSLNs treatment by ∼17% (p < 0.01), but no significant difference in lipid droplets accumulation was found in the Q- and QSLNs-treatment groups. E2 treatment to OVx rats showed a reduction in lipid accumulation by ∼25% (p < 0.001) comparable to the sham group.Effect of quercetin-solid lipid nanoparticles (QSLNs) on bone turnover marker
Bone loss is characterized by an increasing rate of bone turnover due to ovariectomy, and this condition is represented by elevated levels of urinary CTx (collagen breakdown product). Fig. 5C shows that the urinary CTx level was significantly higher in the ovariectomy group by ∼27% (p < 0.001) compared with the sham group, while the animal groups treated with Q, QSLNs and E2 had significantly lower levels of CTx marker by ∼13% (p < 0.05), ∼36% (p < 0.001) and ∼31% (p < 0.001), respectively, when compared with the OVx group. The urinary CTx level of the ovariectomy group treated with QSLNs was comparable with the sham and E2 groups. This data suggest that QSLNs treatment reduced the CTx level by ∼27% (p < 0.001) over the Q group while both inhibit bone loss, which increases under the condition of oestrogen deficiency.Effect of quercetin-solid lipid nanoparticles (QSLNs) on uterine histomorphometry
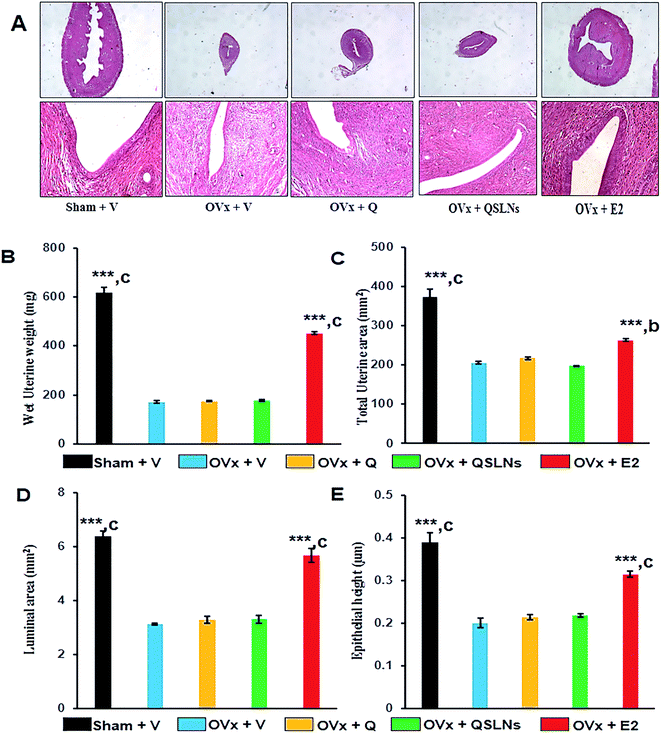
Molecules showing oestrogenicity due to endometrial hyperplasia are characterized by an increase in uterine weight, luminal area and luminal epithelial cell height.66 Fig. 6A shows representative images of the uterus. OVx group, which show a decrease in uterine weight, total uterine area, luminal area and luminal epithelial cell height compared with the sham group (Fig. 6B–E). Assessment of these histomorphometric parameters in the Q and QSLNs groups showed they were significantly decreased when compared with the sham group but were comparable with the OVx group (Fig. 6B–E). It was shown that Q and QSLNs treatment at a dose of 5 mg per kg per day inhibited the uteri changes, thereby eliminating any possibility of endometrial hyperplasic effects.Discussion
Nanoparticulate delivery systems are providing a wide range of therapeutic solutions to various disease areas, either through enhancing the target site localization, improving bioavailability or by many other means based on the disease state and application. Numerous reports are available demonstrating the application of nanoparticles in the treatment of osteoporosis, either by surface functionalization or by modifying the pharmacokinetic properties of the drug. Herein, we have developed a system of quercetin-solid lipid nanoparticles (QSLNs) by using emulsion solvent evaporation followed by a cold homogenization technique. QSLNs of desired size (<200 nm) were obtained at an optimized pressure of 1200 bar at 15 cycles. The size of the QSLNs were optimized below 200 nm and topology confirmation by AFM revealed them to be spherical-shaped particles with a smooth surface. Considering the stability data, it was clear that the developed formulation was stable at 25 °C for at least 3 months and it also showed good stability in SGF/SIF without any significant difference in size and PDI. In vitro release studies demonstrated a better release profile of the QSLNs (∼75%) compared to quercetin (<10%) in 48 h, indicating the direct effect of the increase in surface area of the particles and the formulation composition on the dissolution of quercetin. To evaluate the effect of QSLNs on the bioavailability enhancement, single oral dose pharmacokinetics were performed at a dose of 25 mg kg−1. A nearly 3.5-fold increase in the AUC was observed with a significant change in clearance and t1/2. Here, we used quercetin at a dose of 5 mg per kg per day for treatment following our earlier in vivo study.3,56 This dose has shown positive effects in oestrogen deficiency induced bone loss model of osteoporosis. SLNs are known to improve bioavailability due to a variety of mechanisms. Our data are consistent with the results of Xinxin Yue et al., who reported the in vivo bone regeneration capability of simvastatin-loaded nanoparticles, with a 4.8-fold enhancement of bioavailability compared to a simvastatin suspension.67 Similarly, Hosny et al. showed the advantage of alendronate sodium loaded solid lipid nanoparticles, with an enhanced bioavailability of 7.4-fold along with reduced oesophageal ulceration in rabbits.68 Here, we give several lines of evidence to support our hypothesis that quercetin would increase its preventive efficacy under oestrogen deficiency.It is essential to restore the bone micro-architectural parameters to evaluate the impact of treatment on the quality of trabecular bone, which is metabolically more active and thereby rapidly lost due to oestrogen deficiency in the OVx group. In respect of the restoration of bone microarchitecture, QSLNs treatment was found to be better in distal femoral metaphysis over the Q group. Moreover, bone microarchitecture deterioration was higher in the proximal tibiae metaphysis than in distal femoral metaphysis after OVx.65 Restoration of the bone micro-architectural trabecular parameters in femoral and tibiae metaphysis of osteopenic animals was partial in all the treated groups (Q, QSLNs and E2). However, QSLNs treatment was significantly better than Q treatment and was comparable to the E2 group in all the parameters examined here. In addition, restoration of bone micro-architectural parameters of the L-5 vertebra disclosed significantly better microarchitecture in QSLNs over the Q group. A lower SMI, which indicates a preferred plate-like structure, was found in the L-5 of the QSLNs group over the Q group, advocating a better geometric arrangement of the trabecular bone that would counter compression fracture. It is understood that a nanostructured formulation enables the drug/flavonoid to remain available in a higher concentration and for a longer period. This could be the reason for achieving the enhanced osteogenic efficacy. In a previous report, our lab reported a layer-by-layer (LbL) nanomatrix formulation for the prolonged bioavailability of kaempferol with enhanced retention in bone marrow to achieve better bone formation. A single oral dose (5.0 mg per kg per day) of kaempferol-loaded LbL nanomatrix formulation significantly enhanced the bioavailability when compared to unformulated kaempferol. Formulated kaempferol treatment for 3 months to osteopenic rats showed increased plasma and bone marrow kaempferol levels by 2.8-fold and 1.75-fold, respectively, when compared to free kaempferol treatment. The treatment of formulated kaempferol showed an increased number of bone marrow osteoprogenitor cells, expression of osteogenic genes and bone formation rate as well as a better trabecular microarchitecture. Formulated kaempferol could be a strategy to improve the bioavailability of flavonoids. Considering the basis of the bone regeneration potential of quercetin, in this study we aimed to develop an oral delivery system to enhance the bioavailability of quercetin and its retention in serum to attain enhanced osteoprotective function.56
One of the expected outcomes of the inhibition of the osteoclastic differentiation of BMCs is the prevention of bone loss under oestrogen deficiency.69 Post-ovariectomy bone loss in rats parallels skeletal changes observed in postmenopausal women.70 Here, QSLNs were found to be more potent than Q in inhibiting osteoclastic differentiation from BMCs when we analysed the skeletal effects of Q and QSLNs in the OVx group. Together, the data show that the treatment with QSLNs in the OVx group preserved trabecular BMD at both the axial (L-5) and appendicular bones (femur and tibia) and QSLNs were more effective than Q in preventing bone loss under oestrogen deficiency in animals. The results of the femur three-point bending test revealed that QSLNs treatment was able to prevent the decrease in energy and stiffness of femoral diaphysis in ovariectomy-induced osteopenic condition, while Q was less effective, thus providing evidence of the better preventive effect of QSLNs than Q.
The efficacy to prevent bone loss was greater in QSLNs over Q and this was evident at the cellular and molecular levels. It was found that the QSLNs and Q groups had significantly lower femoral mRNA levels of C-fos, TRAP and RANK compared to the OVx group. Here, it is noticeable that the efficacy to prevent bone loss by QSLNs was comparable to the E2 group. Furthermore, it was also found during in vitro osteoclastogenesis from bone marrow cells that QSLNs and Q prevented osteoclast formation following a lower level of expression of TRAP and RANK mRNA over the control group. QSLNs were found to be better than Q at inhibiting the expression of osteoclast genes, such as TRAP and RANK, in in vitro osteoclastogenesis.
Further evidence of QSLNs action to prevent bone loss included a lowering of the bone turnover marker in the OVx group treated with QSLNs, Q and E2. Bone turnover markers, such as urinary CTx, is an indicator of a higher rate of bone remodelling because the resorption phase is short and the formation phase is long. The higher level of CTx in the OVx group indicated an increase in the rate of bone remodelling. One characteristic of menopause is an acceleration in bone remodelling due to uncoupling between the resorption and formation phase, hence bone resorption by osteoclasts becomes faster than bone formation by osteoblasts, which results in a net bone loss.36 The lower level of CTx in the QSLNs group compared with the OVx group suggested that QSLNs decreased the rate of bone turnover under oestrogen deficiency. Q treatment also decreased the rate of bone turnover, albeit to a lesser extent than QSLNs.
One important concern with the use of phytoestrogens for the treatment of postmenopausal osteoporosis is their potential for uterine endometrial hyperplasia.71,72 Those cells may occasionally become precancerous. Therefore, osteoporosis studies included monitoring participants for uterine endometrial hyperplasia as well.71,72 Assessment of the uterine area, luminal area and luminal epithelial cell height in rat uteri showed the absence of an oestrogen-like action of QSLNs and Q. We proposed that QSLNs are safe (in terms of uterine hyperplasic effects) to treat postmenopausal osteoporosis. Even though we have shown in this study that QSLNs inhibit the function of osteoclasts, which is necessary to prevent bone loss, yet quercetin is also reported to stimulate bone formation.2 Therefore, it comes into view that the better sustainability effect of QSLNs is associated with the decrease in osteoclast differentiation that is achieved by its increased bioavailability and serum retention time.
Conclusions
We have shown that QSLNs at an oral dose of 5 mg per kg per day alleviate the estrogen deficiency induced loss of bone mass, bone strength and the microarchitecture of long bones and vertebrae, eliminating any possibility of endometrial hyperplasic effects. The bone loss preventing effect of QSLNs is significantly better than Q. Application of this QSLNs strategy to deliver other phytoestrogens/drugs needs to be examined further to treat osteoporosis. An alternative therapeutic application of QSLNs could be proposed in the setting of postmenopausal bone loss.Conflict of interest
Authors declare no conflict of interest.Author's contribution
All the authors have contributed in manuscript writing. Final version of the manuscript has been approved by all the authors.Supporting grants
Council of Scientific and Industrial Research, Government of India.Acknowledgements
This work was supported by research grants from CSIR as BSC0201. NA, VT, PK, AK, SS, AKT acknowledge Council of Scientific and Industrial Research (CSIR), Govt. of India for Research Fellowships. AV gratefully acknowledges Indian Council of Medical Research (ICMR), New Delhi, India for the award of research fellowship. NA also thanks to the financial support from “BSC0111 INDPTH (Integrated NextGen approaches in health, disease and Environmental toxicity)” is acknowledged. CDRI communication number for this manuscript was 9335.References
- P. C. H. Hollman and M. B. Katan, Food Chem. Toxicol., 1999, 37, 937–942 CrossRef CAS PubMed
.
- M. Tsuji, H. Yamamoto, T. Sato, Y. Mizuha, Y. Kawai, Y. Taketani, S. Kato, J. Terao, T. Inakuma and E. Takeda, J. Bone Miner. Metab., 2009, 27, 673–681 CrossRef CAS PubMed
.
- J. A. Siddiqui, K. Sharan, G. Swarnkar, P. Rawat, M. Kumar, L. Manickavasagam, R. Maurya, D. Pierroz and N. Chattopadhyay, Menopause, 2011, 18, 198–207 Search PubMed
.
- J. Jakubowicz-Gil, R. Paduch, T. Piersiak, K. Głowniak, A. Gawron and M. Kandefer-Szerszeń, Biochem. Pharmacol., 2005, 69, 1343–1350 CrossRef CAS PubMed
.
- J. Asaum, H. Matsuzaki, S. Kawasak, M. Kuroda, Y. Takeda, K. Kishi and Y. Hiraki, Anticancer Res., 2000, 20, 2477–2483 CAS
.
- L. Čipák, P. Rauko, E. Miadoková, I. Čipáková and L. Novotný, Leuk. Res., 2003, 27, 65–72 CrossRef
.
- M. M. Chan, D. Fong, K. J. Soprano, W. F. Holmes and H. Heverling, J. Cell. Physiol., 2003, 194, 63–70 CrossRef CAS PubMed
.
- R. A. Fearn and B. H. Hirst, Environ. Toxicol. Pharmacol., 2006, 21, 168–178 CrossRef CAS PubMed
.
- R. Gugler, M. Leschik and H. J. Dengler, Eur. J. Clin. Pharmacol., 1975, 9, 229–234 CrossRef CAS PubMed
.
- P. R. Mishra, R. Trivedi, G. K. Gupta, A. Kumar, V. Gupta, S. K. Rath, K. Srivastava and N. Chattopadhyay, Controlled Release Micro-Capsule for osteogenic action, US Pat. 13/203,603, 2013.
- R. H. Müller, K. Mäder and S. Gohla, Eur. J. Pharm. Biopharm., 2000, 50, 161–177 CrossRef
.
- http://www.fda.gov/drugs/informationondrugs/ucm113978.htm.
- S. Kalepu, M. Manthina and V. Padavala, Acta Pharm. Sin. B, 2013, 3, 361–372 CrossRef
.
- N. P. Aditya and S. Ko, RSC Adv., 2015, 5, 30902–30911 RSC
.
- A. zur Mühlen, C. Schwarz and W. Mehnert, Eur. J. Pharm. Biopharm., 1998, 45, 149–155 CrossRef
.
- Y. Luo, D. Chen, L. Ren, X. Zhao and J. Qin, J. Controlled Release, 2006, 114, 53–59 CrossRef CAS PubMed
.
- K. Manjunath and V. Venkateswarlu, J. Controlled Release, 2005, 107, 215–228 CrossRef CAS PubMed
.
- P. Chattopadhyay, B. Y. Shekunov, D. Yim, D. Cipolla, B. Boyd and S. Farr, Adv. Drug Delivery Rev., 2007, 59, 444–453 CrossRef CAS PubMed
.
- R. Sivaramakrishnan, C. Nakamura, W. Mehnert, H. C. Korting, K. D. Kramer and M. Schäfer-Korting, J. Controlled Release, 2004, 97, 493–502 CrossRef CAS PubMed
.
- R. Cavalli, M. R. Gasco, P. Chetoni, S. Burgalassi and M. F. Saettone, Int. J. Pharm., 2002, 238, 241–245 CrossRef CAS PubMed
.
- A. J. Humberstone and W. N. Charman, Adv. Drug Delivery Rev., 1997, 25, 103–128 CrossRef CAS
.
- K. Vandita, B. Shashi, K. G. Santosh and K. I. Pal, Mol. Pharm., 2012, 9, 3411–3421 CrossRef CAS PubMed
.
- H. Li, X. Zhao, Y. Ma, G. Zhai, L. Li and H. Lou, J. Controlled Release, 2009, 133, 238–244 CrossRef CAS PubMed
.
- R. H. Müller, S. A. Runge, V. Ravelli, A. F. Thünemann, W. Mehnert and E. B. Souto, Eur. J. Pharm. Biopharm., 2008, 68, 535–544 CrossRef PubMed
.
- V. V. Kumar, D. Chandrasekar, S. Ramakrishna, V. Kishan, Y. M. Rao and P. V. Diwan, Int. J. Pharm., 2007, 335, 167–175 CrossRef CAS PubMed
.
- G. Huang, N. Zhang, X. Bi and M. Dou, Int. J. Pharm., 2008, 355, 314–320 CrossRef CAS PubMed
.
- K. Ruckmani.
- L. Serpe, M. G. Catalano, R. Cavalli, E. Ugazio, O. Bosco, R. Canaparo, E. Muntoni, R. Frairia, M. R. Gasco, M. Eandi and G. P. Zara, Eur. J. Pharm. Biopharm., 2004, 58, 673–680 CrossRef CAS PubMed
.
- V. Venkateswarlu and K. Manjunath, J. Controlled Release, 2004, 95, 627–638 CrossRef CAS PubMed
.
- G. M. Prelevic and H. S. Jacobs Baillière's, Clin. Endocrinol. Metab., 1997, 11, 311–340 CAS
.
- Y. Ishimi, Clin. Calcium, 2006, 16, 1661–1667 CAS
.
- T. C. Hillard, S. Whitcroft, M. C. Ellerington and M. I. Whitehead, J. Clin. Pharm. Ther., 1991, 16, 231–245 CrossRef CAS PubMed
.
- V. Coxam, Proc. Nutr. Soc., 2008, 67, 184–195 CrossRef PubMed
.
- P. D. Delmas, Journal of Epidemiology and Biostatistics, 1999, 4, 155–160 CAS
.
- B. H. Arjmandi, J. Am. Coll. Nutr., 2001, 20, 417S–420S CrossRef
.
- K. Sharan.
- A. Wattel, S. Kamel, C. Prouillet, J.-P. Petit, F. Lorget, E. Offord and M. Brazier, J. Cell. Biochem., 2004, 92, 285–295 CrossRef CAS PubMed
.
- J.-T. Woo, H. Nakagawa, M. Notoya, T. Yonezawa, N. Udagawa, I.-S. Lee, M. Ohnishi, H. Hagiwara and K. Nagai, Biol. Pharm. Bull., 2004, 27, 504–509 CAS
.
- A. Wattel, S. Kamel, R. Mentaverri, F. Lorget, C. Prouillet, J.-P. Petit, P. Fardelonne and M. Brazier, Biochem. Pharmacol., 2003, 65, 35–42 CrossRef CAS PubMed
.
- D. Rachoń, T. Vortherms, D. Seidlová-Wuttke, H. Jarry and W. Wuttke, Food Chem. Toxicol., 2008, 46, 513–518 CrossRef PubMed
.
- B. H. Arjmandi, R. Birnbaum, N. V. Goyal, M. J. Getlinger, S. Juma, L. Alekel, C. M. Hasler, M. L. Drum, B. W. Hollis and S. C. Kukreja, Am. J. Clin. Nutr., 1998, 68, 1364S–1368S CAS
.
- B. H. Arjmandi, M. J. Getlinger, N. V. Goyal, L. Alekel, C. M. Hasler, S. Juma, M. L. Drum, B. W. Hollis and S. C. Kukreja, Am. J. Clin. Nutr., 1998, 68, 1358S–1363S CAS
.
- R. C. Muhlbauer and F. Li, Nature, 1999, 401, 343–344 CrossRef CAS PubMed
.
- M.-N. Horcajada-Molteni, V. Crespy, V. Coxam, M.-J. Davicco, C. Rémésy and J.-P. Barlet, J. Bone Miner. Res., 2000, 15, 2251–2258 CrossRef CAS PubMed
.
- C. M. Rassi, M. Lieberherr, G. Chaumaz, A. Pointillart and G. Cournot, Cell Tissue Res., 2005, 319, 383–393 CrossRef CAS PubMed
.
- N. Mittapelly, R. Rachumallu, G. Pandey, S. Sharma, A. Arya, R. S. Bhatta and P. R. Mishra, Eur. J. Pharm. Biopharm., 2016, 101, 62–71 CrossRef CAS PubMed
.
- S. Sharma, A. Verma, G. Pandey, N. Mittapelly and P. R. Mishra, Acta Biomater., 2015, 26, 169–183 CrossRef CAS PubMed
.
- M. L. Bouxsein, S. K. Boyd, B. A. Christiansen, R. E. Guldberg, K. J. Jepsen and R. Müller, J. Bone Miner. Res., 2010, 25, 1468–1486 CrossRef PubMed
.
- K. Srivastava, K. Khan, A. M. Tyagi, M. P. Khan, D. K. Yadav, R. Trivedi, R. Maurya, D. Singh and N. Chattopadhyay, J. Evidence-Based Complementary Altern. Med., 2013, 2013, 12 Search PubMed
.
- R. Trivedi, A. Kumar, V. Gupta, S. Kumar, G. K. Nagar, J. R. Romero, A. K. Dwivedi and N. Chattopadhyay, Mol. Cell. Endocrinol., 2009, 302, 86–91 CrossRef CAS PubMed
.
- T. O. R. Hildebrand and P. Rüegsegger, Comput. Meth. Biomech. Biomed. Eng., 1997, 1, 15–23 CrossRef PubMed
.
- D. Ulrich, B. van Rietbergen, A. Laib and P.
![[R with combining umlaut]](https://www.rsc.org/images/entities/char_0052_0308.gif) uegsegger, Bone, 1999, 25, 55–60 CrossRef CAS PubMed
uegsegger, Bone, 1999, 25, 55–60 CrossRef CAS PubMed .
- A. Odgaard and H. J. G. Gundersen, Bone, 1993, 14, 173–182 CrossRef CAS PubMed
.
- G. K. Gupta, A. Kumar, V. Khedgikar, P. Kushwaha, J. Gautam, G. K. Nagar, V. Gupta, A. Verma, A. K. Dwivedi, A. Misra, R. Trivedi and P. R. Mishra, Nanomedicine, 2013, 8, 757–771 CrossRef CAS PubMed
.
- V. Khedgikar, J. Gautam, P. Kushwaha, A. Kumar, G. K. Nagar, P. Dixit, R. Chillara, S. Voruganti, S. P. Singh, W. Uddin, G. K. Jain, D. Singh, R. Maurya, N. Chattopadhyay and R. Trivedi, Menopause, 2012, 19, 1336–1346 Search PubMed
.
- A. Kumar, G. K. Gupta, V. Khedgikar, J. Gautam, P. Kushwaha, B. Changkija, G. K. Nagar, V. Gupta, A. Verma, A. K. Dwivedi, N. Chattopadhyay, P. R. Mishra and R. Trivedi, Eur. J. Pharm. Biopharm., 2012, 82, 508–517 CrossRef CAS PubMed
.
- K. E. Heim, A. R. Tagliaferro and D. J. Bobilya, J. Nutr. Biochem., 2002, 13, 572–584 CrossRef CAS PubMed
.
- R. Santiago-Mora, A. Casado-Díaz, M. D. De Castro and J. M. Quesada-Gómez, Osteoporosis Int., 2011, 22, 675–684 CrossRef CAS PubMed
.
- R. Trivedi, S. Kumar, A. Kumar, J. A. Siddiqui, G. Swarnkar, V. Gupta, A. Kendurker, A. K. Dwivedi, J. R. Romero and N. Chattopadhyay, Mol. Cell. Endocrinol., 2008, 289, 85–93 CrossRef CAS PubMed
.
- S. Bandyopadhyay, J.-M. Lion, R. Mentaverri, D. A. Ricupero, S. Kamel, J. R. Romero and N. Chattopadhyay, Biochem. Pharmacol., 2006, 72, 184–197 CrossRef CAS PubMed
.
- K. V. Sashidhara, M. Kumar, V. Khedgikar, P. Kushwaha, R. K. Modukuri, A. Kumar, J. Gautam, D. Singh, B. Sridhar and R. Trivedi, J. Med. Chem., 2013, 56, 109–122 CrossRef CAS PubMed
.
- H. Z. Ke, H. K. Chen, H. A. Simmons, H. Qi, D. T. Crawford, C. M. Pirie, K. L. Chidsey-Frink, Y. F. Ma, W. S. S. Jee and D. D. Thompson, Bone, 1997, 20, 31–39 CrossRef CAS PubMed
.
- G. Swarnkar, K. Sharan, J. A. Siddiqui, J. S. Mishra, K. Khan, M. P. Khan, V. Gupta, P. Rawat, R. Maurya, A. K. Dwivedi, S. Sanyal and N. Chattopadhyay, Br. J. Pharmacol., 2012, 165, 1526–1542 CrossRef CAS PubMed
.
- A. Kumari, S. K. Yadav, Y. B. Pakade, B. Singh and S. C. Yadav, Colloids Surf., B, 2010, 80, 184–192 CrossRef CAS PubMed
.
- T. J. Wronski, L. M. Dann and S. L. Horner, Bone, 1989, 10, 295–301 CrossRef CAS PubMed
.
- F. J. Möller, P. Diel, O. Zierau, T. Hertrampf, J. Maaß and G. Vollmer, Toxicol. Lett., 2010, 196, 142–153 CrossRef PubMed
.
- Y. Xinxin, N. Mao, Z. Te, W. Cheng, W. Zhonglei, W. Wangxi, Z. Qi, L. Chunhua and Z. Lei, Nanotechnology, 2016, 27, 115708 CrossRef PubMed
.
- K. M. Hosny, PLoS One, 2016, 11, e0154926 Search PubMed
.
- P. Taxel, H. Kaneko, S.-K. Lee, H. L. Aguila, L. G. Raisz and J. A. Lorenzo, Osteoporosis Int., 2008, 19, 193–199 CrossRef CAS PubMed
.
- S. A. Jelinsky, S. E. Choe, J. S. Crabtree, M. M. Cotreau, E. Wilson, K. Saraf, A. J. Dorner, E. L. Brown, B. J. Peano, X. Zhang, R. C. Winneker and H. A. Harris, BMC Med. Genomics, 2008, 1, 1–12 CrossRef PubMed
.
- J. I. Macgregor and V. C. Jordan, Pharmacol. Rev., 1998, 50, 151–196 CAS
.
- M. Neves-E-Castro, Clin. Obstet. Gynecol., 2008, 51, 607–617 CrossRef PubMed
.
Footnote |
| † Both the authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |