Comparison of titania nanotube-supported cobalt catalysts prepared by impregnation and homogeneous precipitation for Fischer–Tropsch synthesis
Juan Li,
Tiejun Wang,
Liangpeng Wu and
Xinjun Li*
Key Laboratory of Renewable Energy, Guangzhou Institute of Energy Conversion, Chinese Academy of Sciences, Guangzhou 510640, China. E-mail: lixj@ms.giec.ac.cn
First published on 16th September 2016
Abstract
The properties and catalytic performance of Co/TiO2 nanotubes prepared by impregnation (Imp) and homogeneous precipitation (HP) for Fischer–Tropsch synthesis were investigated. HP makes cobalt particles enrich the exterior surface of the support, while for the Imp catalyst, cobalt particles are distributed on both external and internal surfaces of the support. The HP catalyst displays a twofold higher activity as well as superior C5+ selectivity. The low activity of the Imp catalyst can be attributed to the strong Co–TiO2 interaction and less available active cobalt sites.
1. Introduction
Fischer–Tropsch synthesis (FTS) is a promising way to produce clean liquid fuels and valuable chemicals via syngas (CO and H2) derived from coal, natural gas and biomass.1–3 Cobalt-based catalysts are used widely in the commercial FTS process because of their high activity and selectivity to long-chain linear hydrocarbons, and low activity for the competitive water–gas shift reaction.4,5The activity of cobalt catalysts for FTS depends on the number of cobalt metal atoms on the surface. To gain high metal dispersion and improve their catalytic performance in FTS, cobalt is typically deposited on supports with high surface area, such as silica,6,7 alumina,8,9 titania,9,10 zeolites11–13 and carbon materials.14,15 Recently, some ordered mesoporous materials (e.g. SBA-15 and HSM) were also studied as the support to prepare cobalt catalysts.16–18 However, the strong interaction between cobalt and support often makes the reduction of cobalt oxide difficult, suppresses the formation of active cobalt metal species and thereby limits the activity of supported cobalt catalysts. Various efforts have been done to improve the FTS performance of cobalt catalysts. It is reported that the addition of small amount of metal ions including noble metals,19,20 alkali metals,21 alkali earth metals,22 rare earth elements23 could enhance the reducibility and dispersion of active cobalt metal species. On the other hand, loading method also influences the structure of cobalt metal and the activity of cobalt catalysts. Synthesis methods for supported cobalt catalysts include impregnation,24,25 deposition precipitation,25,26 co-precipitation,27 sol–gel,28 chemical vapor deposition,29 and solvothermal method30 and so on. To obtain highly active and selective FTS catalysts, Co/SiO2 and Co/Al2O3 catalysts are usually synthesized by incipient wetness impregnation method. Since the physical and chemical properties of support materials play an important role in altering the FTS performance, the optimum preparation method should vary with the kinds of support materials.
TiO2 as support is suitable for the application of FTS reaction due to low cost, safety and chemical stability.9,10 Compared to normal TiO2 particles, TiO2 nanotubes have higher surface area, which is more benefit for the dispersion of active cobalt species. Our previous study found that Fe2O3 nanoparticles encapsulated in TiO2 nanotubes presented an excellent yield of oil phase hydrocarbons and C5+ selectivity due to the confinement effect.31 However, the research on TiO2 nanotube supported cobalt catalysts for FTS has been rarely reported. In this work, we compared properties and FTS performance of Co/TiO2 nanotube catalysts prepared by impregnation (Imp) and homogenous precipitation (HP) methods. The correlation between the structure of cobalt and the catalytic performance of Co/TiO2 nanotubes was investigated in order to obtain the catalyst design concept.
2. Experimental
2.1 Catalyst preparation
Titanate nanotubes were prepared by a hydrothermal method according to our previous report.32 The fresh prepared powder of titanate nanotubes (4 g) was dispersed in deionized water and the pH was adjusted to 4 with acetic acid solution. Then tetraethoxysilane (TEOS, 0.8 mL) ethanol solution was slowly added into the mixture and stirred for 5 h. After filtration, the powder was washed with ethanol and H2O for several times to remove residual ion and dried at 343 K. Finally, the powder was calcined at 873 K for 2 h to get the modified TiO2 nanotubes (named as TNT).TNT supported cobalt catalyst was prepared with a cobalt loading of 15 wt%. For Imp method, TNT was impregnated by an aqueous solution of Co(NO3)2·6H2O with the incipient wetness volume (1.0 mL g−1). Then the sample was dried at 343 K overnight and calcined at 673 K for 4 h (named as Co/TNT-Imp). For HP method, Co(NO3)2·6H2O (1.5 g), TNT (2 g) and urea (15 g) were dispersed to 200 mL of distilled water, followed by heating at 363 K for 15 h under magnetic stirring and reflux. The suspension was filtered and washed with distilled water. The obtained powder was dried at 343 K overnight and calcined at 673 K for 4 h (named as Co/TNT-HP).
2.2 Catalyst characterization
The actual loading of cobalt was measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES) on a Plasma-Spec-I spectrometer. 10 mg of sample was dissolved in a mixture of nitric acid and hydrofluoric acid at 353 K, and the solution was diluted to 100 mL with deionized water. Power X-ray diffraction (XRD) pattern was recorded using a PANalytical X'Pert Pro diffractometer with Cu Kα radiation source operated at 40 kV and 40 mA. Surface area (SA) and porosity were analyzed by N2 adsorption–desorption using a SI-MP-10 automated system at 77 K after the samples were degassed in a vacuum at 473 K for 6 h. The morphology of the sample was observed using a FEI Tecnai G20 high-resolution electron microscopy (HRTEM). X-ray photoelectron spectroscopy (XPS) was determined using a Thermo ESCALAB 250XI with monochromatized Al Kα source (1486.6 eV). The C 1s line was taken as an internal standard at 284.8 eV. Hydrogen temperature-programmed reduction (H2-TPR) was performed using a mixture gas of 5% H2/95% Ar (v/v) with a flow of 50 mL min−1. About 30 mg of catalyst was heated from room temperature to 1173 K at a heating rate of 10 K min−1. Hydrogen chemisorption experiments were carried out on Chemstart. 300 mg of catalyst was reduced at 673 K for 5 h and then cooled to 373 K under 9.85% H2/Ar flow. Afterwards, the flow of H2/Ar was switched to argon at the same temperature, which lasted for 1 h in order to remove the physisorbed hydrogen. The temperature programmed desorption (TPD) of the sample was obtained by increasing the temperature to 1173 K under argon flow. The reduction degree of supported cobalt was calculated from the amount of H2 consumption during H2 reduction pretreatment at 673 K for 5 h. The amount of desorbed hydrogen was calculated by comparing to the area of mean calibration pulses of H2 in argon.2.3 Catalytic activity test
The FTS reaction was conducted in a stainless steel fixed bed reactor. The prepared catalyst (1.0 g) was mixed with 1.0 g of quartz granules and reduced in a flow of high purity H2 at 673 K for 12 h. After reduction, the catalyst was cooled to 423 K in flowing H2. Then, the syngas (H2/CO = 2, space velocity of 3000 mL g−1 h−1) was introduced and the pressure was increased to 2.0 MPa. After that, the reaction temperature was slowly increased to 523 K and maintained for 48 h. The tail gas was analyzed online by gas chromatograph (GC, Agilent, 7890 A) equipped with a TCD and a FID. The liquid products in the trap were collected and analyzed offline using a Shimadzu GC-2010. N2 in the feed gas was used as internal standard to calculate CO conversion and hydrocarbon selectivity.3. Results and discussion
The actual loading of cobalt determined by ICP is 15.7 wt% for the Co/TNT-HP and 15.4 wt% for the Co/TNT-Imp, respectively. The two catalysts have a nearly same Co/Ti atom ratio with a value of about 0.21. The crystal structures of the Co/TNT-HP and the Co/TNT-Imp catalysts are determined by XRD as illustrated in Fig. 1a. Diffraction lines assigned to anatase TiO2 (PDF#: 21-1272) and Co3O4 (PDF#: 42-1467) are observed from the XRD patterns of two catalysts. Peaks of other cobalt compounds such as CoO and Co–titanate are not observed. Crystallite size of Co3O4 is estimated from the diffraction line at 2θ = 36.8° using the Scherrer equation. The crystallite size of Co3O4 is 14.8 nm for the Co/TNT-HP and 12.6 nm for the Co/TNT-Imp, respectively. The crystallite size of Co3O4 over the Co/TNT-Imp is much smaller compared to the Co/TNT-HP catalyst. | ||
| Fig. 1 XRD patterns (a) and pore size distributions (b) of the synthesized samples. Black triangle and circle show Co3O4 and anatase TiO2, respectively. | ||
Surface area, average pore size and pore volume of the synthesized samples were measured by N2 adsorption, as listed in Table 1. The TNT support has a surface area of 230 m2 g−1 and a pore volume of 0.85 cm3 g−1. For the Co/TNT-HP and the Co/TNT-Imp catalysts after loading cobalt, the surface areas are decreased to 187 m2 g−1 and 154 m2 g−1, and the pore volumes are decreased to 0.76 cm3 g−1 and 0.57 cm3 g−1, respectively. The Co/TNT-HP and the Co/TNT-Imp exhibit a broad pore-size distribution and peaks at the range of 4–20 nm with the center at about 9.5 nm as shown in Fig. 1b, which could be attributed to the inner cavity of TiO2 nanotubes.31–33 For the Co/TNT-Imp, the surface area and pore volume are clearly lower than the TNT support. This may be due to the fact that a part of cobalt particles enter into the pores of TiO2 nanotubes during the impregnation preparation and take up some of the pore volume.
| Sample | Coa (wt%) | SAb (m2 g−1) | Vporeb (cm3 g−1) | Dporeb (nm) | Co3O4 size (nm) | Co/Ti atom ratio | H2 uptakec μmol H2/g | DRedd (%) | DCoe (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| XRD | TEM | ICP | XPS | ||||||||
| a The Co loading were measured by ICP.b The texture data were estimated using the desorption branch of the isotherm.c H2 chemisorption temperature was 373 K.d Reduction degree of Co.e Dispersion of Co metal was calculated assuming the stoichiometry Had/Cos = 1. | |||||||||||
| TNT | — | 230 | 0.85 | 9.6 | — | — | — | — | |||
| Co/TNT-HP | 15.7 | 187 | 0.76 | 9.6 | 14.8 | 15.3 | 0.213 | 0.57 | 294.6 | 88.9 | 13.5 |
| Co/TNT-Imp | 15.4 | 154 | 0.57 | 9.5 | 12.6 | 10.8 | 0.209 | 0.12 | 302.3 | 79.3 | 14.0 |
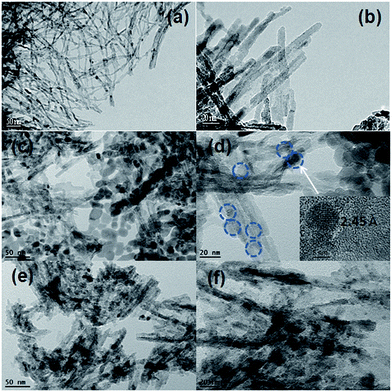
Fig. 2 shows TEM images of the TNT support, the Co/TNT-HP and the Co/TNT-Imp catalysts. It is observed from TEM images in Fig. 2a and b that the TNT remains one-dimensional nano-tubular morphology after calcined at 873 K. For the Co/TNT-HP catalyst, Co3O4 particles are mostly well dispersed on the surface of TNT support as indicated by Fig. 2d. HRTEM image in the inset of Fig. 2d recorded from the marked white arrow region shows the clear crystal lattices with interplanar distance of 2.45 Å corresponding to the Co3O4 (311) plane. The Co/TNT-HP catalyst exhibits an average Co3O4 size of about 15.3 nm, which is in good accordance with the crystallite size obtained from XRD line broadening. Besides, a few isolated unsupported Co3O4 particles with larger size were also found (Fig. 2c), which can be related to bulk precipitation during urea hydrolysis. In case of the Co/TNT-Imp catalyst, a lot of Co3O4 particle clusters are observed from TEM images. Meantime, wide areas of the TNT support are empty (Fig. 2e and f). This may be explained as a consequence of the deposition of a part of cobalt precursor within the inner cavity of TNT due to the capillary effect during the catalyst preparation, as has been reported for the Fe particles encapsulated in TiO2 and carbon nanotubes prepared via the impregnation method.31,34 Co particles over the Co/TNT-Imp have an average size of about 10.8 nm, much smaller than those over the Co/TNT-HP. The small particle size of Co3O4 on the Co/TNT-Imp may be due to the strong cobalt–support interaction.
The reduction behavior of the catalysts was studied by H2-TPR, as shown in Fig. 3. The TPR profiles of two catalysts are characterized by three main reduction stages, showing complex features with overlapping H2 consumption peaks. For the Co/TNT-HP catalyst, the first peak (603–723 K) is associated with the reduction of Co3O4 to CoO, while the second one (723–903 K) corresponds to the reduction of CoO to metallic cobalt.25,35 The third peak (903–1123 K) is the reduction of cobalt species with strong Co–TiO2 interaction.35 In case of the Co/TNT-Imp catalyst, the reduction peaks are shifted to higher temperature, with the first peak (Co3O4 to CoO) at 603–763 K and the second one at 763–1043 K. The second peak features a distinct broadening, which can be attributed to the reduction of CoO to metallic cobalt and cobalt species with interaction. These results show that the reducibility of cobalt on the Co/TNT-HP is much higher than that on the Co/TNT-Imp, further indicating that the cobalt–support interaction on the former is much weak than that on the latter. Similar finding has been reported on the Co/Al2O3 catalyst prepared by impregnation and homogeneous precipitation.26
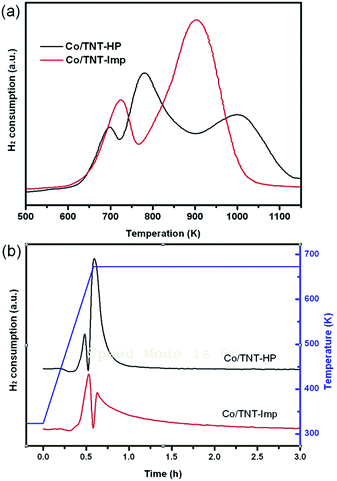 | ||
| Fig. 3 H2-TPR profiles of the synthesized catalysts obtained by heating from ambient to 1173 K (a), and heating from ambient to 673 K and keeping at 673 K for 5 h (b). | ||
The reduction degree of Co (DRed) and the dispersion of Co metal (DCo) were summarized in Table 1. The reduction degree was calculated from H2-TPR profiles obtained by holding the H2 reduction temperature at 673 K for 5 h, as shown in Fig. 3b. The reduction degrees of Co on the CO/TNT-HP and Co/TNT-Imp catalysts are 89.9% and 79.3%, respectively. The Co/TNT-HP shows the higher reduction degree compared to the Co/TNT-Imp, which is agreement with the TPR results in Fig. 3a. This indicates that the moderate Co–support interaction over the Co/TNT-HP would promote the reduction of Co oxide species. A balanced interaction between the support and the active phase is particularly important for FTS.2 The dispersion of Co metal was measured by H2 chemisorption and calculated based on the total amount of Co atoms in sample. The dispersion of Co metal over the Co/TNT catalysts is about 14% and not largely influenced by the Co-loading method. Results of XRD, TEM, H2-TPR and H2 chemisorption clearly show that Co particles with small size and low reducibility are formed by the Imp method but those with the moderate Co–support interaction and high reducibility are formed by the HP method.
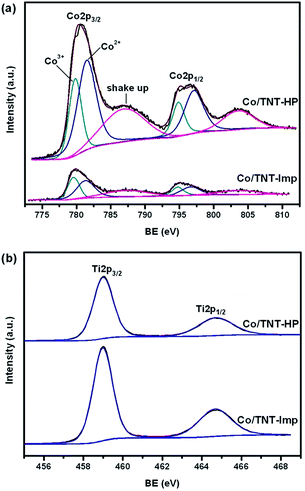
The surface chemical composition and oxide state of the as-prepared Co/TNT catalysts were determined by XPS. The XPS spectra of Co 2p and Ti 2p recorded are shown in Fig. 4. Two characteristic broad peaks containing the Co2+ and Co3+ 2p3/2 states at about 780.2 eV and the Co2+ and Co3+ 2p1/2 states at about 795.6 eV were observed from the Co peaks of two catalysts. The binding energy between the Co 2p1/2 and Co 2p3/2 peaks is 15.4 eV, which is in agreement with the standard Co3O4 phase.13 Furthermore, both the Co 2p3/2 and Co 2p1/2 peaks contain a shake-up satellite peak, which is associated with the presence of Co2+,13,36 particularly evident for the Co/TNT-HP catalyst. The interaction of metal–support may change the electronic state of the active metal.2 Generally, the weak interaction between cobalt oxide and support is prone to form lower valence state cobalt.27 It is deduced from the XPS analysis that the Co2+ components on the surface of the Co/TNT-HP are much higher compared to the Co/TNT-Imp, indicating a weaker Co–TiO2 interaction, which was also proved by the results of TPR. The surface atomic ratio of Co/Ti determined by XPS was 0.57 for the Co/TNT-HP and 0.12 for the Co/TNT-Imp, respectively. This further indicates that the cobalt particles are enriched on the exterior surface of TNT for the Co/TNT-HP, but are distributed on both external and internal surfaces of TNT for the Co/TNT-Imp, as verified by TEM analysis.
The FTS performances are examined over the Co/TNT-HP and Co/TNT-Imp catalysts under the temperature of 523 K and pressure of 2.0 MPa for 48 h. The catalytic reaction results obtained are given in Table 2 and Fig. 5. The preparation method significantly affects the activity and selectivity of the obtained catalysts. The two catalysts display a relatively stable activity under the given reaction condition (Fig. 6), indicating that the structure of cobalt would hardly change before and after FT reaction. The Co/TNT-Imp exhibits a CO conversion of about 31.0%. For the Co/TNT-HP, CO conversion is about 74.2%, two fold higher than the Co/TNT-Imp. Combining with the results of structural analysis by TPR and XPS, the lower activity over the Co/TNT-Imp is mainly due to the strong Co–TiO2 interaction and less available active cobalt sites. The balanced interaction between the support and the active phase is particularly important for FTS.2 The Co/TNT-Imp catalyst prepared by the Imp method presents smaller cobalt particle size than that of the catalyst prepared by the HP method. Small particle size tends to show stronger Co–support interaction, which will cause difficulty in the reduction of the precursor of the active phase and thus decreases the activity of FTS reaction. Another possible reason for the reduced activity of the Co/TNT-Imp catalyst could be explained as follows: a part of cobalt particles are embedded in the inner cavity of TNT and the diffusion of reactants (CO and H2) to active cobalt sites is restricted. For the Co/TNT-HP catalyst, adsorption of the cobalt ions onto the support surface coincides with nucleation and growth of the precursor compound in the deposition process via the homogeneous precipitation method, which will lead to a proper Co–support interaction and hence exhibit a superior FTS activity.
| Catalysts | CO conversion (%) | CO2 selectivity (mol%) | Hydrocarbon selectivity (wt%) | C5+ yield (mg gcat−1 h−1) | |||
|---|---|---|---|---|---|---|---|
| CH4 | C2–C4 | C5+ | Olefin | ||||
| a Reaction conditions: P = 2.0 MPa, T = 523 K, H2/CO = 2, space velocity was 3000 ml g−1 h−1. | |||||||
| Co/TNT-HP | 74.2 | 1.7 | 26.3 | 11.1 | 62.6 | 8.6 | 242 |
| Co/TNT-Imp | 31.0 | 1.9 | 38.2 | 30.3 | 31.5 | 1.4 | 46 |
The C5+ selectivity over the Co/TNT-HP is 62.6%, which is much higher than 31.5% over the Co/TNT-Imp. The yield of liquid hydrocarbon (C5+) over the Co/TNT-HP is 242 mg gcat−1 h−1, which is about five times as much as that of the Co/TNT-Imp (46 mg gcat−1 h−1). It is notable that the Co/TNT-Imp exhibits a high selectivity for CH4 (38.2%) and C2–C4 short-chain hydrocarbon (30.3%). As previously reported, the catalyst containing small Co particles tends to produce lighter hydrocarbons.8 In this study, the Co/TNT-Imp catalyst contains smaller cobalt particles compared to the Co/TNT-HP catalyst, contributing to the formation of more methane and short-chain hydrocarbon.
4. Conclusions
The titania nanotube supported cobalt catalysts were prepared by impregnation and homogeneous precipitation and examined for the FTS reaction. The preparation method has a significant impact on the activity and selectivity of the obtained catalysts. The Co/TNT-HP provides higher conversion of CO as well as selectivity of C5+ product. For comparison, the Co/TNT-Imp exhibits a low CO conversion and produces lots of methane and C2–C4 short-chain hydrocarbon. The low activity of the Co/TNT-Imp catalyst is attributed to the strong Co–TiO2 interaction and less available active cobalt sites.Acknowledgements
Thanks for the support financially by the National Natural Science Foundation of China (51302263), the National Key Basic Research Program of China (2013CB228105) and Guangdong Natural Science Foundation (2015A030313715).Notes and references
- H. Schulz, Appl. Catal., A, 1999, 186, 3 CrossRef CAS.
- Q. H. Zhang, J. C. Kang and Y. Wang, ChemCatChem, 2010, 2, 1030 CrossRef CAS.
- G. W. Huber, S. Iborra and A. Corma, Chem. Rev., 2006, 106, 4044 CrossRef CAS PubMed.
- A. Y. Khodakov, W. Chu and P. Fongarland, Chem. Rev., 2007, 107, 1692 CrossRef CAS PubMed.
- E. Iglesia, Appl. Catal., A, 1997, 161, 59 CrossRef CAS.
- S. Sun, N. Tsubaki and K. Fujimoto, Appl. Catal., A, 2000, 202, 121 CrossRef CAS.
- P. R. Karandikar, Y. J. Lee, G. Kwak, M. H. Woo, S. J. Park, H. G. Park, K. S. Ha and K. W. Jun, J. Phys. Chem. C, 2014, 118, 21975 CAS.
- J. Y. Park, Y. J. Lee, P. R. Karandikar, K. W. Jun, K. S. Ha and H. G. Park, Appl. Catal., A, 2012, 411–412, 15 CrossRef CAS.
- S. Storsæter, B. Tøtdal, J. C. Walmsley, B. S. Tanem and A. Holmen, J. Catal., 2005, 36, 139 CrossRef.
- K. Jalama, J. Kabuba, H. Xiong and L. L. Jewell, Catal. Commun., 2012, 17, 154 CrossRef CAS.
- Q. Tang, Q. Zhang, P. Wang, Y. Wang and H. Wan, Chem. Mater., 2004, 16, 1967 CrossRef CAS.
- C. Xing, G. Yang, M. Wu, R. Yang, L. Tan, P. Zhu, Q. Wei, J. Li, J. Mao and Y. Yoneyama, Fuel, 2015, 148, 48 CrossRef CAS.
- L. Wu, Z. Li, D. Han, J. Wu and D. Zhang, Fuel Process. Technol., 2015, 134, 449 CrossRef CAS.
- T. Fu and Z. Li, Chem. Eng. Sci., 2015, 135, 3 CrossRef CAS.
- Y. Zhu, Y. Ye, S. Zhang, M. E. Leong and F. Tao, Langmuir, 2012, 28, 8275 CrossRef CAS PubMed.
- A. Martínez, C. López, F. Márquez and I. Díaz, J. Catal., 2003, 220, 486 CrossRef.
- Y. Lu, P. Zhou, J. Han and F. Yu, RSC Adv., 2015, 5, 59792 RSC.
- D. Yin, W. Li, W. Yang, H. Xiang, Y. Sun, B. Zhong and S. Peng, Microporous Mesoporous Mater., 2001, 47, 15 CrossRef CAS.
- W. Ma, G. Jacobs, R. A. Keogh, D. B. Bukur and B. H. Davis, Appl. Catal., A, 2012, 437–438, 1 CrossRef CAS.
- T. O. Eschemann, J. Oenema and K. P. de Jong, Catal. Today, 2016, 261, 60 CrossRef CAS.
- U. Cornaro, S. Rossini, T. Montanari, E. Finocchio and G. Busca, Catal. Today, 2012, 197, 101 CrossRef CAS.
- T. Miyazawa, T. Hanaoka and S. Hirata, Catal. Today, 2014, 232, 2 CrossRef.
- Y. Zhang, K. Liew, J. Li and X. Zhan, Catal. Lett., 2010, 139, 1 CrossRef CAS.
- N. Osakoo, R. Henkel, S. Loiha, F. Roessner and J. Wittayakun, Catal. Commun., 2015, 66, 73 CrossRef CAS.
- K. Shimura, T. Miyazawa, T. Hanaoka and S. Hirata, Appl. Catal., A, 2014, 475, 1 CrossRef CAS.
- T. O. Eschemann, J. H. Bitter and K. P. de Jong, Catal. Today, 2014, 228, 89 CrossRef CAS.
- W. G. Zhou, J. Y. Liu, X. Wu, J. F. Chen and Y. Zhang, Catal. Commun., 2015, 60, 76 CrossRef CAS.
- Y. Wang, B. Hou, J. Chen, L. Jia, D. Li and Y. Sun, Catal. Commun., 2009, 10, 747 CrossRef CAS.
- A. T. Najafabadi, A. A. Khodadadi, M. J. Parnian and Y. Mortazavi, Appl. Catal., A, 2016, 511, 31 CrossRef.
- R. Xie, D. Li, B. Hou, J. Wang, L. Jia and Y. Su, Catal. Commun., 2011, 12, 380 CrossRef CAS.
- W. Wang, M. Ding, L. Ma, X. Yang, J. Li, N. Tsubaki, G. Yang, T. Wang and X. Li, Fuel, 2016, 164, 347 CrossRef CAS.
- J. Li, L. Wu, L. Long, M. Xi and X. Li, Appl. Surf. Sci., 2014, 322, 265 CrossRef CAS.
- L. Long, X. Yu, L. Wu, J. Li and X. Li, Nanotechnology, 2014, 25, 035603 CrossRef PubMed.
- W. Chen, Z. L. Fan, X. L. Pan and X. H. Bao, J. Am. Chem. Soc., 2008, 130, 9414 CrossRef CAS PubMed.
- S. Mu, D. Li, B. Hou, J. Chen and Y. Sun, Catal. Lett., 2009, 133, 341 CrossRef CAS.
- Q. Wang, Y. Peng, J. Fu, G. Z. Kyzas, S. M. R. Billah and S. An, Appl. Catal., B, 2015, 168–169, 42 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |