Grafting modification of epoxidized natural rubber with poly(ethylene glycol) monomethylether carboxylic acid and ionic conductivity of graft polymer composite electrolytes
Ran Wang,
Hua Mei,
Wentan Ren* and
Yong Zhang
School of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, China
First published on 2nd November 2016
Abstract
A novel comb-like polymer was synthesized via grafting epoxidized natural rubber (ENR) with polyethylene glycol (PEG) monomethylether carboxylic acid (mPEG-COOH), and the grafting reaction was studied by variable-temperature Fourier transform infrared (FTIR) spectroscopy. This comb-like polymer was characterized by attenuated total reflectance-FTIR (ATR-FTIR) spectroscopy and differential scanning calorimetry (DSC). The carboxyl groups of mPEG-COOH reacted with the epoxy groups of ENR to produce graft polymers. The composite polymer electrolyte (CPE) based on this comb-like polymer ENR-g-mPEG-COOH was prepared via introducing LiClO4 into the graft polymer matrix. The CPE was studied by X-ray diffraction analysis (XRD), DSC, an equilibrium swelling method, and electrochemical workstation techniques. With increasing mPEG-COOH content, the ionic conductivity of the electrolyte significantly increased.
1 Introduction
Polymer composite electrolytes (PEs) have attracted abundant research interest in the past few decades.1–4 Enjoying a high capacity to dissolve lithium salts and ionic conductivity above 70 °C, polyethylene oxide (PEO) is the most studied polymer electrolyte matrix.2,5–7 However, the conductivity of PEO is still limited by its inherent crystallization at room temperature.8,9 Researchers went off the beaten track and designed rubbers as polymer matrices. Elastomers, especially rubber, are notable for their excellent elasticity, low glass transition temperature (Tg) and soft elastomeric nature at room temperature.10 Many modification methods based on elastomers, such as introducing grafting11 and crosslinking structure,12 and adding inorganic fillers,1,13,14 have already been successfully applied in obtaining new polymer electrolytes.Epoxidized natural rubber (ENR), a modified form of natural rubber, has good potential to become a polymer host in CPE because of its distinctive characteristic such as low Tg, soft elastomeric characteristics at room temperature15 and good electrode–electrolyte adhesion. The liquid ENR and poly(vinyl)chloride (PVC) blends are also used as a host for PEs.4 In ENR-based polymer electrolytes, the highly flexible macromolecular chains and the polar epoxy groups of ENR provide excellent segmental motion, coordination sites for Li+ transport and reactivity with other groups like carboxyl and amine groups.16,17 Furthermore, ENR also offers good contact between electrolytic layer and electrode for batteries.18
mPEG-COOH is a carboxylated compound of polyethylene glycol mono methyl ether. The ether oxygen groups on the polyethylene glycol chain have coordination reactivity with the cation to make it dissolve, and have the best space coordination to form a homogeneous system. Therefore, polyethylene glycol is widely used in polymeric electrolytes. A new PEG-based PEs is developed for quasi-solid-state battery application.19 However, a large amounts of liquid electrolytes is needed in this system, which might be evaporated at high temperature and thus would lower the conductivity.20 Hence, in order to obtain more stable and highly ionic conductive polymer electrolytes, modifying polymer is necessary.
This paper mainly studied the graft modification between ENR 50 and mPEG-COOH, which could inhibit the crystallization for mPEG-COOH and improve the conductivity of CPE. The grafting reaction and the structure of graft polymer ENR-g-mPEG-COOH was investigated by variable temperature infrared spectroscopy (FTIR), ATR-FTIR and DSC. By adding lithium salt LiClO4, crosslinked polymer electrolyte ENR-g-mPEG-COOH/LiClO4 was also prepared by the coordination effect. The CPE were characterized by DSC, XRD and the equilibrium swelling method. The effect of mPEG-COOH content on the ionic conductivity was also discussed.
2 Experimental
2.1 Materials
The ENR used was epoxidized natural rubber with 50% epoxidation and the trade name is ENR 50, produced by the Chinese Academy of Tropical Agricultural Sciences (CATAS, Hainan, China), and its structure was shown in Fig. 1(a). mPEG-COOH (98.8%, Mw = 2000) was purchased from YaRe Biological Technology Co. Ltd (Shanghai, China), and its structure was shown in Fig. 1(b).All solvents including tetrahydrofuran (THF), N,N-dimethylformamide (DMF), methanol, toluene and methanol were supplied by Sinopharm Reagent Co. Ltd, China.
2.2 Sample preparation
ENR 50 was first dissolved in THF and then precipitated in methanol for purification. The precipitated ENR was washed with methanol for several times before vacuum dried at 40 °C for 24 h. ENR 50 solutions in DMF (70 mL) containing 5, 10, 15, 20, 30, 40, 60 and 80 phr (per hundred rubber by weight) mPEG-COOH were stirred mechanically in oil bath at 140 °C for 8 h in nitrogen atmosphere and then rotary evaporation was used to remove the excess solvent. Then the product was vacuum dried at 80 °C for 12 h. The samples were extracted with methanol for 24 h, and then the purified products were dried in a vacuum oven at 25 °C for 3 days. Therefore, graft polymer ENR-g-mPEG-COOH was obtained as a CPE matrix.The graft polymer ENR-g-mPEG-COOH was dissolved in THF (30 mL), then LiClO4 (40 phr) was added and stirring for 3 h. The solution was poured into Teflon mould, and the solvent was evaporated at room temperature. Further drying of the solutions was then conducted in a vacuum oven at 40 °C for 24 h. Finally, the CPE films were successfully prepared.
2.3 Characterization and instruments
In situ FTIR spectroscopy. FTIR spectra of ENR/mPEG-COOH mixture were recorded to study the process of the grafting reaction. ENR 50 and mPEG-COOH (100/80) dissolved in THF were dropped on KBr pellets and transferred to a temperature-controlled cell in an FTIR spectrometer (NEXUS 470). Testing temperature increased from 40 to 170 °C at a heating rate of 5 °C min−1. FTIR spectra were recorded when the temperature increased by every 10 °C. Spectra were obtained in the range 400–4000 cm−1 with four scans at a resolution of 4 cm−1.
ATR-FTIR spectroscopy. ATR-FTIR analysis was carried out using a Perkin-Elmer Spectrum 100 in the wavenumber range of 4000 to 650 cm−1 at room temperature.
Differential scanning calorimetry (DSC). Grafted products of ENR-g-mPEG-COOH were tested by DSC, TA Q2000 instrument (TA instrument, USA). ENR-g-mPEG-COOH matrix were sealed in hermetic aluminum pans and scanned at a heating of 20 °C min−1 under a nitrogen flow. In each case, the following cycle was used: cooling at a rate of 20 °C min−1 from room temperature to −50 °C, heating at a rate of 20 °C min−1 to 120 °C, cooling at 10 °C min−1 to −50 °C and heating at the same rate of 20 °C min−1 up to 100 °C.
The ENR-g-mPEG-COOH/LiClO4 CPE are tested in the same method.
The equilibrium swelling method. The crosslink density of the ENR-g-mPEG-COOH/LiClO4 CPE was determined by the equilibrium swelling method.21 The samples were first weighed to calculate their initial dry weight (m1), then they were inserted into bottles containing 50 mL toluene at 23 °C for three days until swelling equilibrium. Solvent on the surfaces of the samples were removed, then samples were weighed to determine their equilibrium weight (m2). The samples then were dried in an oven under 60 °C for 24 hours and reweighed to determine the final dry weight (m3). The crosslinking density was calculated from the Flory–Rehner equation:22
 | (1) |
In eqn (1), νe is the crosslink density (in mole per unit volume), ν is the molar volume of the solvent, ν2 is the volume fraction of the polymer in the swollen mass and calculated according to eqn (2), and χ is the Flory–Huggins polymer–solvent interaction parameter calculated with eqn (3),
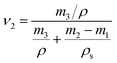 | (2) |
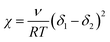 | (3) |
XRD measurements. XRD measurements were carried out at room temperature on a D/MAX-2200/PC diffractometer (Rigaku Co. Ltd, Japan), using the Cu Kα radiation at 40 kV/40 mA for 2θ values between 5° and 60° with a scanning speed of 6° per min.
Ionic conductivity. Ionic conductivity was measured by alternating current complex impedance analysis using Auto-lab PGSTA302 electrochemical test system at room temperature. The frequency ranged from 1 MHz to 1 Hz and the signal amplitude was 10 mV. The polymer solid electrolyte was sandwiched between the stainless steel ion-blocking electrodes with a surface contact area of 1 cm2. The conductivity (σ) can be calculated from the eqn (4),23–25
 | (4) |
3 Results and discussion
3.1 In situ FTIR spectroscopy
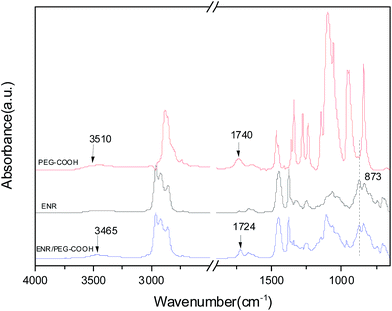
In situ FTIR spectra of ENR/mPEG-COOH (100/80) compound is shown in Fig. 2. ENR 50 has the same absorption peaks with the basic characteristics of natural rubber, such as C–H stretching mode at 2960 and 2920 cm−1,26,27 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching mode at 1651 cm−1 and CH2 scissoring mode at 1451 cm−1.28 The characteristic absorption peak of ENR 50 is the C–O–C stretching mode of epoxy ring at 873 cm−1. Using the absorption peak at 873 cm−1 to evaluate the content of epoxy groups is well documented.29,30 The characteristic absorption peaks corresponding to the carboxyl group of mPEG-COOH at 1740 and 948 cm−1 are attributed to the free carboxyl C
C stretching mode at 1651 cm−1 and CH2 scissoring mode at 1451 cm−1.28 The characteristic absorption peak of ENR 50 is the C–O–C stretching mode of epoxy ring at 873 cm−1. Using the absorption peak at 873 cm−1 to evaluate the content of epoxy groups is well documented.29,30 The characteristic absorption peaks corresponding to the carboxyl group of mPEG-COOH at 1740 and 948 cm−1 are attributed to the free carboxyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O stretching vibrations, respectively. Peaks at 873 cm−1 (epoxy groups in ENR 50) and 1740 cm−1 (C
O and C–O stretching vibrations, respectively. Peaks at 873 cm−1 (epoxy groups in ENR 50) and 1740 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretch of carboxyl groups in mPEG-COOH) gradually disappear, new peaks at 3510 cm−1 (stretching vibrations of O–H hydroxyl groups) and 1728 cm−1 (C
O stretch of carboxyl groups in mPEG-COOH) gradually disappear, new peaks at 3510 cm−1 (stretching vibrations of O–H hydroxyl groups) and 1728 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of ester bonds) are observed with increasing temperature. This implies that ENR 50 has reacted with mPEG-COOH to form ENR-g-mPEG-COOH.
O of ester bonds) are observed with increasing temperature. This implies that ENR 50 has reacted with mPEG-COOH to form ENR-g-mPEG-COOH.
 | ||
| Fig. 2 In situ FTIR spectra of ENR 50/mPEG-COOH compound (40–170 °C): (a) 4000–1500 cm−1 (b) 1600–800 cm−1. | ||
The process of grafting reaction of ENR 50 and mPEG-COOH was investigated by the intensity changes of absorption peaks at 873, 1740, 1728 and 3470 cm−1. As the samples are unchanged during the reaction, the conversion of the epoxy group and the carboxyl group could be directly characterized by the area of these absorption peaks. The conversion was calculated based on Lambert–Beer law which is shown as eqn (5).
 | (5) |
According to actual situation, the formula was converted to
 | (6) |
As shown in Fig. 3, the conversion of epoxy group and carboxyl group increased significantly with temperature increasing from 130 °C to 170 °C, indicating the grafting reaction took place between epoxy and carboxyl groups.
Fig. 4(a) and (b) show the relative intensity of the carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and hydroxyl O–H absorption peak at different temperatures, which are determined by the changes of their peak areas. Fig. 4(a) shows that the absorption peak intensity of the free carboxyl groups (1740 cm−1) gradually decreases with increasing temperature. The hydrogen bonded carbonyl absorption peak intensity (1664 cm−1) declines linearly with the increment of temperature until the absorption peak disappears at 140 °C, and the peak position shifts to 1728 cm−1 which is corresponding to ester carbonyl group. The absorption intensity (1728 cm−1) increases rapidly, which indicates that carboxyl group participate in the reaction to generate ester bond. As shown in Fig. 4(b), with the increment of temperature, the peak intensity of hydrogen bond gradually decreases at 140 °C, meanwhile, free hydroxyl groups at 3510 cm−1 begins to appear. In addition, the intensity of the absorption peak of the alcohol hydroxyl (3510 cm−1) increases as temperature increases. It is evident that consumption of carboxyl groups (3470 and 1740 cm−1) is accompanied by the formation of hydroxyl groups (3510 cm−1) and C
O and hydroxyl O–H absorption peak at different temperatures, which are determined by the changes of their peak areas. Fig. 4(a) shows that the absorption peak intensity of the free carboxyl groups (1740 cm−1) gradually decreases with increasing temperature. The hydrogen bonded carbonyl absorption peak intensity (1664 cm−1) declines linearly with the increment of temperature until the absorption peak disappears at 140 °C, and the peak position shifts to 1728 cm−1 which is corresponding to ester carbonyl group. The absorption intensity (1728 cm−1) increases rapidly, which indicates that carboxyl group participate in the reaction to generate ester bond. As shown in Fig. 4(b), with the increment of temperature, the peak intensity of hydrogen bond gradually decreases at 140 °C, meanwhile, free hydroxyl groups at 3510 cm−1 begins to appear. In addition, the intensity of the absorption peak of the alcohol hydroxyl (3510 cm−1) increases as temperature increases. It is evident that consumption of carboxyl groups (3470 and 1740 cm−1) is accompanied by the formation of hydroxyl groups (3510 cm−1) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups of ester bonds (1728 cm−1), which further confirms that the reaction between epoxy groups and carboxyl groups takes place during grafting.
O groups of ester bonds (1728 cm−1), which further confirms that the reaction between epoxy groups and carboxyl groups takes place during grafting.
3.2 Characterization of graft polymer ENR-g-mPEG
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), respectively. Fig. 6 shows the ATR-FTIR spectra of ENR 50 grafted with varying content of mPEG-COOH. The absorption peak of epoxy ring mode at 873 cm−1 decreases with increasing of mPEG-COOH content. The absorption peaks at 1724 cm−1 and 1110 cm−1 also increase, which respectively correspond to ester bond and ether bond. And there are two different peaks in CPE (80 phr) from others: 1280 and 1344 cm−1. Peak at 1344 cm−1 is attributed to a blend of ring δCH and of δOCH and δCCH in trans-OCH2CH2O. This indicating the gauche-OCH2CH2O– segments presumably in amorphous state. The emergence of new peak reveals that some (smaller) fraction of the C
O), respectively. Fig. 6 shows the ATR-FTIR spectra of ENR 50 grafted with varying content of mPEG-COOH. The absorption peak of epoxy ring mode at 873 cm−1 decreases with increasing of mPEG-COOH content. The absorption peaks at 1724 cm−1 and 1110 cm−1 also increase, which respectively correspond to ester bond and ether bond. And there are two different peaks in CPE (80 phr) from others: 1280 and 1344 cm−1. Peak at 1344 cm−1 is attributed to a blend of ring δCH and of δOCH and δCCH in trans-OCH2CH2O. This indicating the gauche-OCH2CH2O– segments presumably in amorphous state. The emergence of new peak reveals that some (smaller) fraction of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester group has become bonded in a different environment.31 The new bands at 1280 cm−1 correspond to the C–O stretching vibration.32 We can see the same peaks in spectra of mPEG-COOH in Fig. 5. The peak of 1280 cm−1 is different from others. I think that might be no complete reaction to mPEG-COOH and some residues in the mixture.
O ester group has become bonded in a different environment.31 The new bands at 1280 cm−1 correspond to the C–O stretching vibration.32 We can see the same peaks in spectra of mPEG-COOH in Fig. 5. The peak of 1280 cm−1 is different from others. I think that might be no complete reaction to mPEG-COOH and some residues in the mixture.
FT-IR spectroscopic analysis results demonstrate that more carboxyl group react with epoxy group with increasing of mPEG-COOH. The grafting reaction mechanism is shown in Scheme 1.
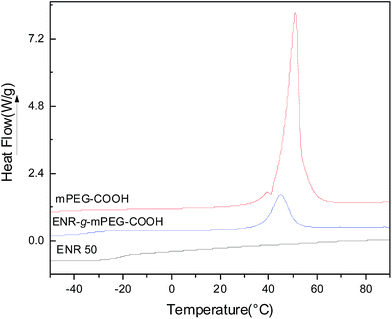
Fig. 7 shows the DSC curves of reactants and grafting products after extraction. Table 1 presents a summary of the main data from the curves. The relative crystallinity (Xc) could be calculated from the eqn (7).
 | (7) |
| Samples | Tg (°C) | Tm (°C) | ΔHm (J g−1) | Xc (%) |
|---|---|---|---|---|
| ENR 50 | −21.0 | — | — | — |
| ENR-g-mPEG (100/80) | −31.1 | 44.8 | 32.8 | 15.3 |
| mPEG-COOH | — | 51.0 | 146.4 | 68.5 |
In which  is the melting enthalpy of a completely crystalline mPEG-COOH sample.33 As shown in Fig. 7, the grafting of mPEG-COOH to ENR 50 causes the decreasing of Tg by 10 °C, indicating a greater fraction of –OCH2CH2O– units contributes to a lower Tg. Compared with mPEG-COOH, the melting peak of ENR-g-mPEG-COOH shifts to lower temperature. Meanwhile, the crystallinity of ENR-g-mPEG-COOH decreases from 68.5% to 15.3%, which indicates the crystallization of mPEG-COOH is inhibited and the crystalline size decreases when mPEG-COOH is grafted to ENR 50. The polymer chain segment moves more easily due to the introduction of mPEG and the increment of amorphous region. One of the main reasons is that the introduction of flexible side chains leads to an increment of the free volume of the polymer chains. The reduction of crystallinity and the decrement of Tg are beneficial to improve the ionic conductivity of CPE.
is the melting enthalpy of a completely crystalline mPEG-COOH sample.33 As shown in Fig. 7, the grafting of mPEG-COOH to ENR 50 causes the decreasing of Tg by 10 °C, indicating a greater fraction of –OCH2CH2O– units contributes to a lower Tg. Compared with mPEG-COOH, the melting peak of ENR-g-mPEG-COOH shifts to lower temperature. Meanwhile, the crystallinity of ENR-g-mPEG-COOH decreases from 68.5% to 15.3%, which indicates the crystallization of mPEG-COOH is inhibited and the crystalline size decreases when mPEG-COOH is grafted to ENR 50. The polymer chain segment moves more easily due to the introduction of mPEG and the increment of amorphous region. One of the main reasons is that the introduction of flexible side chains leads to an increment of the free volume of the polymer chains. The reduction of crystallinity and the decrement of Tg are beneficial to improve the ionic conductivity of CPE.
3.3 The characterization of ENR-g-mPEG-COOH/LiClO4 CPE
 | ||
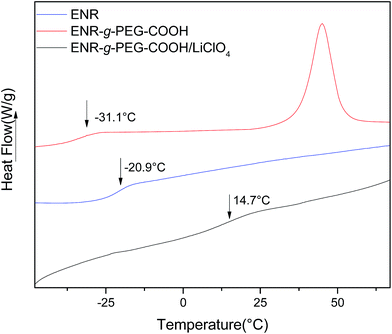
| Fig. 9 DSC thermographs of pure ENR 50, and ENR-g-mPEG-COOH/LiClO4 CPE with various content of mPEG-COOH. | ||
| mPEG-COOH (phr) | Tg (°C) |
|---|---|
| 0 | −20.9 |
| 20 | 21.7 |
| 40 | 18.7 |
| 60 | 16.3 |
| 80 | 14.7 |
 | (8) |
The swelling ratio and crosslink density of ENR-g-mPEG-COOH/LiClO4 CPE are shown in Fig. 10. The lithium salt LiClO4 was added into ENR-g-mPEG-COOH to introduce a crosslinking reaction to CPE. Lithium ion and polymer produced coordination crosslinking to make composite polymer swell without dissolving. With the increase of mPEG-COOH content, the swelling ratio decreases and crosslink density increases. The results indicate that the crosslink density of CPE increase with the increment of mPEG-COOH contents. The Li+ coordinate with more oxygen atoms so that the crosslinking network is improved and the swelling ratio of CPE decreased.
3.4 Ionic conductivity
Fig. 12 shows the impedance spectra of the ENR-g-mPEG-COOH/LiClO4 CPE at 25 °C as a function of mPEG-COOH content. The composition dependence of the CPE ionic conductivity is presented in Fig. 13 for better interpretation. The conductivity of CPE increases as the weight fraction of mPEG-COOH increases partly due to an increment of free Li+ carrier quantity and decrement of polymer chain entanglement within the test range. When the weight ratio of mPEG-COOH to ENR 50 is 0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the ionic conductivity reaches the maximum of 8.93 × 10−5 S cm−1. This is because the graft reaction between mPEG-COOH and ENR brings flexible chain segments to the polymer matrix, thus improves the interaction ability with lithium salt. The directional movement of Li+ is achieved in the electric field. With the increments of mPEG-COOH contents, the conductivity of ENR-g-mPEG-COOH/LiClO4 CPE will increase.
1, the ionic conductivity reaches the maximum of 8.93 × 10−5 S cm−1. This is because the graft reaction between mPEG-COOH and ENR brings flexible chain segments to the polymer matrix, thus improves the interaction ability with lithium salt. The directional movement of Li+ is achieved in the electric field. With the increments of mPEG-COOH contents, the conductivity of ENR-g-mPEG-COOH/LiClO4 CPE will increase.
 | ||
| Fig. 12 A.C. impedance spectra of the ENR-g-mPEG-COOH/LiClO4 solid electrolyte composites with different mPEG-COOH content: (a) from 5 phr to 80 phr, (b) 40 phr to 80 phr. | ||
The simplified equivalent circuit model was constructed to analyze the impedance spectra in Fig. 14. The Rs illustrates the resistive movement of charge carriers in CPE. A Rct and the constant phase element (Qdl) are assigned to the charge transfer resistance and to the double layer capacitance, respectively. Zw is the diffusion Warburg impedance. The element of Qdl, Rct, and Zw arises from the double layer at the electrolyte interface in the system. The capacitive character of the double layer at the is denoted by a constant phase element instead of an ideal capacitor. In the impedance spectra, the high-frequency semicircle represents the Li+ embedded firstly transfer-across the activity SEI (solid electrolyte interface) films of the electrode surface; the low frequency line represents the active Li+ diffuse in the electrode. According to the equivalent circuit and the impedance spectra, the CPEs follow a common conduction mechanism. In CPE, two process may occur in the interface region, the charge carries, typically Li+ ions hop along the polar site. They transfer across the interface itself and formation of double layers due to the development of potential drop at electrode surface, which would introduce Li+ concentration gradients in the electrolyte. The Li+ would be driven to move in bulk electrode or out into the electrolyte by the concentration gradients.1,3,37
4 Conclusions
ENR-g-mPEG-COOH graft polymer was successfully prepared via a graft reaction between ENR 50 and mPEG-COOH. The process of grafting reaction was investigated by in situ FTIR. FTIR result indicates that the carboxyl group of mPEG-COOH can react with the epoxy group of ENR 50 to generate the ENR-g-mPEG-COOH composites. DSC analysis shows that the melting temperature, the enthalpy of fusion, and the normalized crystallinity of grafted product were significantly lower than that of mPEG-COOH. In addition, ENR-g-mPEG-COOH/LiClO4 composites electrolyte were prepared through introducing LiClO4 into the ENR-g-mPEG-COOH graft system and the CPE was studied by DSC, the equilibrium swelling method and XRD. The increment of Tg and the analysis of crosslink density can also prove the formation of Li+ and polymer coordination crosslinking. The ionic conductivity of CPE increases with raising mPEG-COOH content. The ionic conductivity reaches the maximum of 8.93 × 10−5 S cm−1. And the mechanism of Li+ transportation in the CPE is explained by the equivalent circuit.Acknowledgements
The authors would like to extend their sincere appreciation to the National Nature Science Foundation of China (Grant No. 51373095).Notes and references
- W. L. Tan and M. Abu Bakar, Ionics, 2016, 22, 1319–1335 CrossRef CAS.
- C. H. Chan, H.-W. Kammer, L. H. Sim, S. N. H. M. Yusoff, A. Hashifudin and T. Winie, Ionics, 2014, 20, 189–199 CrossRef CAS.
- W. L. Tan, M. Abu Bakar and N. H. H. Abu Bakar, Ionics, 2013, 19, 601–613 CrossRef CAS.
- T. K. Lee, A. Ahmad, Y. Farina, H. M. Dahlan and M. Y. A. Rahman, J. Appl. Polym. Sci., 2012, 126, E159–E165 CrossRef CAS.
- B. Sun, P. Tehrani, N. D. Robinson and D. Brandell, J. Mater. Sci., 2013, 48, 5756–5767 CrossRef CAS.
- S. Guinot, E. Salmon, J. Penneau and J. Fauvarque, Electrochim. Acta, 1998, 43, 1163–1170 CrossRef CAS.
- L. Y. Yang, D. X. Wei, M. Xu, Y. F. Yao and Q. Chen, Angew. Chem., Int. Ed., 2014, 53, 3631–3635 CrossRef CAS PubMed.
- N. Boden, S. Leng and I. Ward, Solid State Ionics, 1991, 45, 261–270 CrossRef CAS.
- M. Marzantowicz, J. Dygas, F. Krok, A. Łasińska, Z. Florjańczyk, E. Zygadło-Monikowska and A. Affek, Electrochim. Acta, 2005, 50, 3969–3977 CrossRef CAS.
- L. TianKhoon, N. Ataollahi, N. H. Hassan and A. Ahmad, J. Solid State Electrochem., 2016, 20, 203–213 CrossRef CAS.
- Q. Lin, Br. J. Pharmacol., 2015, 5, 437–444 CrossRef.
- M. Li, W. Ren, Y. Zhang and Y. Zhang, J. Polym. Res., 2012, 19, 1–7 CrossRef.
- Y. Lu, Q. Lin, W. Ren and Y. Zhang, J. Polym. Res., 2015, 22, 1–10 CrossRef CAS.
- Z. Zhu, M. Hong, D. Guo, J. Shi, Z. Tao and J. Chen, J. Am. Chem. Soc., 2014, 136, 16461–16464 CrossRef CAS PubMed.
- F. Latif, M. Aziz, A. M. M. Ali and M. Z. A. Yahya, Macromol. Symp., 2009, 277, 62–68 CrossRef CAS.
- R. Idris, M. D. Glasse, R. J. Latham, R. G. Linford and W. S. Schlindwein, J. Power Sources, 2001, 94, 206–211 CrossRef CAS.
- M. L. Hallensleben, S. H. Rüdiger and R. H. Schuster, J. Histochem. Cytochem., 1995, 227, 643–652 Search PubMed.
- F. Harun and C. H. Chan, in Flexible and Stretchable Electronic Composites, ed. D. Ponnamma, K. K. Sadasivuni, C. Wan, S. Thomas and M. Al-Ali AlMa'adeed, Springer International Publishing, Cham, 2016, pp. 37–59, DOI:10.1007/978-3-319-23663-6_2.
- W. Huang, Z. Zhu, L. Wang, S. Wang, H. Li, Z. Tao, J. Shi, L. Guan and J. Chen, Angew. Chem., Int. Ed., 2013, 52, 9162–9166 CrossRef CAS PubMed.
- E. Quartarone and P. Mustarelli, Chem. Soc. Rev., 2011, 40, 2525–2540 RSC.
- J.-S. Chen, C. K. Ober, M. D. Poliks, Y. Zhang, U. Wiesner and C. Cohen, Polymer, 2004, 45, 1939–1950 CrossRef CAS.
- P. J. Flory and J. Rehner, J. Chem. Phys., 1943, 11, 521–526 CrossRef CAS.
- L. J. Goujon, A. Khaldi, A. Maziz, C. Plesse, G. T. Nguyen, P.-H. Aubert, F. Vidal, C. Chevrot and D. Teyssié, Macromolecules, 2011, 44, 9683–9691 CrossRef CAS.
- J. Luo, A. H. Jensen, N. R. Brooks, J. Sniekers, M. Knipper, D. Aili, Q. Li, B. Vanroy, M. Wubbenhorst, F. Yan, L. Van Meervelt, Z. Shao, J. Fang, Z.-H. Luo, D. E. De Vos, K. Binnemans and J. Fransaer, Energy Environ. Sci., 2015, 8, 1276–1291 CAS.
- X. Chen, H. Tang, T. Putzeys, J. Sniekers, M. Wubbenhorst, K. Binnemans, J. Fransaer, D. E. De Vos, Q. Li and J. Luo, J. Mater. Chem. A, 2016, 4, 12241–12252 CAS.
- E. Mertzel and J. L. Koenig, in Epoxy Resins and Composites II, Springer, 1986, pp. 73–112 Search PubMed.
- S. Noor, A. Ahmad, I. Talib and M. Rahman, Ionics, 2010, 16, 161–170 CrossRef CAS.
- Q. Lin, Y. Lu, W. Ren and Y. Zhang, RSC Adv., 2015, 5, 90031–90040 RSC.
- H. Dannenberg and W. Harp Jr, Anal. Chem., 1956, 28, 86–90 CrossRef CAS.
- N. Zainal, R. Idris and M. Nor Sabirin, Adv. Mater. Res., 2011, 287, 424–427 Search PubMed.
- M. Urbanová, J. Šubrt, A. Galíková and J. Pola, Polym. Degrad. Stab., 2006, 91, 2318–2323 CrossRef.
- M. Laskoski, M. B. Schear, A. Neal, D. D. Dominguez, H. L. Ricks-Laskoski, J. Hervey and T. M. Keller, Polymer, 2015, 67, 185–191 CrossRef CAS.
- X. Li and S. Hsu, J. Polym. Sci., Polym. Phys. Ed., 1984, 22, 1331–1342 CrossRef CAS.
- S. N. H. M. Yusoff, S. L. Har, C. C. Han, A. Hashifudin and H.-W. Kammer, Polym. Res. J., 2013, 7, 159 CAS.
- A. Ahmad, M. Y. b. A. Rahman and I. A. Talib, Nat. Sci., 2010, 2, 190 Search PubMed.
- R. Baskaran, S. Selvasekarapandian, N. Kuwata, J. Kawamura and T. Hattori, Solid State Ionics, 2006, 177, 2679–2682 CrossRef CAS.
- J. H. Lee and K. J. Kim, Electrochim. Acta, 2013, 102, 196–201 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |