Modulating the release of Escherichia coli in double W1/O/W2 emulsion globules under hypo-osmotic pressure†
Abstract
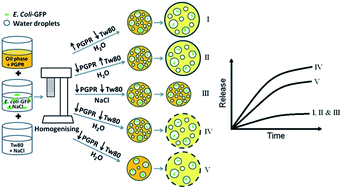
Double water-in-oil-in-water (W1/O/W2) emulsions have the potential to encapsulate bacteria and their structure if destabilised can facilitate bacterial release in a time controlled manner. The description of release mechanisms of bacteria in W1/O/W2 emulsions is limited to hyper-osmotic conditions; however, the mechanism responsible for bacterial release in hypo-osmotic conditions is different and the presence of bacteria may affect the emulsion and vice versa. In this work, the stability and release properties of W1/O/W2 emulsions were studied with or without the presence of GFP-tagged Escherichia coli (E. coli-GFP) in the inner aqueous phase (W1). The impact of altering the osmotic balance by diluting the emulsion in a hypo-osmotic solution was also determined. W1/O/W2 emulsion preparation and E. coli-GFP encapsulation was achieved using a two-step homogenisation process and structure was changed by altering the concentration of hydrophilic surfactant (Tween80, 0.5–10%), lipophilic surfactant (PGPR, 1–8%) and W1 (20% and 40%). The release of E. coli-GFP was monitored by culture and observed using fluorescence microscopy. The release of E. coli-GFP was significantly (P < 0.05) increased when the osmotic balance was altered and at high amounts of W1 and low concentrations of Tween80 or PGPR. Bacterial release occurred due to oil globule bursting and the viability of bacteria was unaffected by the release mechanism. The concentration of bacteria affected the double emulsion structure during the first step of emulsification but had no effect on emulsion structure during osmotic balance alteration.

 Please wait while we load your content...
Please wait while we load your content...