Pre-coating with protein fractions inhibits nano-carrier aggregation in human blood plasma†
Abstract
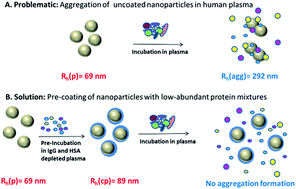
The success of a nanomaterial designed for biomedical applications depends strongly on its “biological identity” meaning the physicochemical properties the material adopts after contact with a physiological medium e.g. blood. Critical issues are here the size stability of the nanomaterial against aggregation in blood induced by blood proteins and the composition of the protein corona. These factors further determine the particles' fate in vivo and in vitro. While this has been seen to occur inevitably we demonstrate here that a preformed and hereby predetermined protein corona steers the nanomaterials behavior concerning aggregation in human blood plasma and uptake behavior in macrophages. Fractionation of human blood plasma was applied to enrich human serum albumin (HSA), immunoglobulin G (IgG) as well as various low abundant protein mixtures. The exact composition of these protein fractions was analyzed via quantitative, label-free liquid-chromatography mass-spectrometry (LC-MS). The protein fractions were further applied to form a predetermined protein corona on differently functionalized polystyrene nanoparticles. The change of the nanoparticles' physicochemical properties after incubation with the defined protein fractions or whole human plasma was studied by dynamic light scattering (DLS) to determine size changes. DLS was also used to investigate the stability of the protein-coated nanoparticles when reintroduced in human plasma. In addition, we found that cellular uptake of nanomaterials was strongly influenced by the artificially created protein corona.

 Please wait while we load your content...
Please wait while we load your content...