DOI:
10.1039/C6RA16975A
(Paper)
RSC Adv., 2016,
6, 74683-74690
Molecular sieve mediated sequential Knoevenagel condensation/decarboxylative Michael addition reaction: efficient and mild conditions for the synthesis of 3,3-disubstituted oxindoles with an all carbon quaternary center†
Received
1st July 2016
, Accepted 1st August 2016
First published on 2nd August 2016
Abstract
A one-pot, molecular sieve mediated sequential Knoevenagel condensation/decarboxylative Michael addition reaction has been developed. We discovered that the molecular sieves not only promoted the Knoevenagel condensation of isatins and malononitrile to generate isatylidene malononitriles, but also promoted the later decarboxylative Michael addition reactions of β-ketoacids and malonic acid half thioesters (MAHTs) with isatylidene malononitriles. This protocol provides a mild and efficient method for the preparation of 3,3-disubstituted oxindoles with an all carbon quaternary center in high yields.
Introduction
The 3,3-disubstituted oxindole framework is a common scaffold found in a wide array of natural products and pharmaceuticals, which show a broad spectrum of intriguing biological activities.1 As a consequence, the privileged structure of 3,3-disubstituted oxindoles bearing complex molecular architectures and functional diversity has attracted synthetic interest. As shown in Fig. 1, amedalin shows an antidepressant effect, and was synthesized in the 1970s.2 Linopiridine is an anti-Alzheimer drug candidate in phase III clinical trials.3 Recently, (phenylpiperazinylbutyl)oxindoles, which are selective 5-HT7 receptor antagonists, have been synthesized and reported.4 Owing to the important pharmaceutical value, numerous elegant methodologies for the synthesis of 3,3-disubstituted oxindoles have been developed.5
 |
| | Fig. 1 Representative drug candidates containing 3,3-disubstituted oxindole framework. | |
In recent years, isatylidene malononitriles, which are isatin derived Michael acceptors, have been applied in the synthesis of 3,3-disubstituted oxindoles with an all-carbon quaternary center. Aliphatic ketones,6 enones,7 1,3-diketones,8 phosphites,9 α,α-dicyanoalkenes,10 amides11 and enol ethers12 have been documented as nucleophiles in the organocatalytic conjugate addition reactions to isatylidene malononitriles. However, the Michael addition reactions of aromatic ketones and thioesters with isatylidene malononitriles are less explored.13 The lower reactivity of both nucleophiles poses a potential problem. We envisioned the decarboxylative Michael addition of β-ketoacids and malonic acid half thioesters (MAHTs) to this Michael acceptor might be a solution to this problem.
However, the existing literature procedures show that most decarboxylative addition reactions require a base, a metal complex or an organic molecule serving as catalyst. Recently, Lu and co-workers developed a catalyst-free, molecular sieve mediated decarboxylative Mannich reaction of β-ketoacids with sulfonyl imines.14 This protocol also promoted decarboxylative aldol reaction between β-ketoacids and isatins. Inspired by this work, we discovered that the molecular sieve not only promoted the Knoevenagel condensation of isatins and malononitrile to generate isatylidene malononitriles, but also promoted the later decarboxylative Michael addition reaction of β-ketoacids and MAHTs with isatylidene malononitriles.
This one-pot protocol meets the 12 Principles of Green Chemistry15 and provides the access to a broad range of 3,3-disubstituted oxindoles in high yields. To the best of our knowledge, there are no precedent methods for the preparation of 3,3-disubstituted oxindoles with an all carbon quaternary center under catalyst-free conditions. We herein wish to report our preliminary results about this work.
Results and discussion
To initiate the investigation, we carried out the model reaction between isatin 1a, malononitrile 2 and β-ketoacid 3a, and the results are summarized in Table 1. The rate for both step 1 and step 2 were quite slow when no additive was added, and only trace amount of 4a was detected by TLC analysis (Table 1, entry 1). To our delight, the reaction proceeded smoothly when 4 Å molecular sieves were added as an additive, and the product 4a was isolated in 94% yield (Table 1, entry 2). Reducing the loading of 4 Å molecular sieves from 300 to 100 and 50 (mg mmol−1) led to a slight decrease in the product yield (Table 1, entries 3 and 4). We then turned our attention to the solvent screening and found EtOAc to be the ideal solvent (Table 1, entries 5–8). The screening of drying agents showed the 4 Å molecular sieves were the utmost efficient. It seems the basicities of drying agents (4 Å > 3 Å > 5 Å > Na2SO4) play a crucial role for the high yield of this reaction (Table 1, entries 9–11). These results were consistent with the report from Lu and co-workers.14 We also found SiO2 and Al2O3 had catalytic activities for this reaction although longer reaction times for step 2 and lower yields were observed (Table 1, entries 12 and 13).
Table 1 Optimization of reaction conditionsa
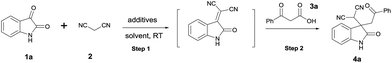
|
| Entry |
Additive |
Loading (mg mmol−1) |
Solvent |
Yieldb (%) |
| Unless otherwise noted, the reaction was carried out by using 0.1 mmol of 1a, 1.2 equiv. of 2, 30 mg 4 Å MS and 1.2 equiv. of 3 in 1 mL of solvent at RT for 12 h (step 1) and 3 h (step 2). Isolated yields. Reaction time for step 2: 72 h. Reaction time for step 2: 24 h. Silicycle P60, 40–63 μm, 230–400 mesh. MN, neutral. |
| 1 |
None |
— |
EtOAc |
Trace |
| 2 |
4 Å MS |
300 |
EtOAc |
94 |
| 3 |
4 Å MS |
100 |
EtOAc |
88 |
| 4 |
4 Å MS |
50 |
EtOAc |
81 |
| 5 |
4 Å MS |
300 |
CH3CN |
93 |
| 6 |
4 Å MS |
300 |
THF |
84 |
| 7 |
4 Å MS |
300 |
Acetone |
78 |
| 8 |
4 Å MS |
300 |
CH2Cl2 |
89 |
| 9 |
3 Å MS |
300 |
EtOAc |
66 |
| 10c |
5 Å MS |
300 |
EtOAc |
51 |
| 11c |
Na2SO4 |
300 |
EtOAc |
<20 |
| 12c |
SiO2e |
300 |
EtOAc |
50 |
| 13d |
Al2O3f |
300 |
EtOAc |
70 |
With the optimized conditions in hand, the general substrate scope of both isatins 1 and β-ketoacids 3 were examined, and the results are shown in Table 2.
Table 2 One-pot reaction of isatins, malononitrile and β-ketoacidsa
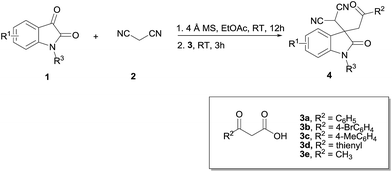
|
| Entry |
R1 |
R2 |
R3 |
4 |
Yieldb (%) |
| Unless otherwise noted, the reaction was carried out by using 0.1 mmol of 1a, 1.2 equiv. of 2, 30 mg 4 Å MS and 1.2 equiv. of 3 in 1 mL of solvent at RT for 12 h (step 1) and 3 h (step 2). Isolated yields. 2 equiv. of 2 at 50 °C for 18 h. 3.6 equiv. of 3b was used. |
| 1 |
H |
C6H5 |
H |
4a |
94 |
| 2c |
4-Cl |
C6H5 |
H |
4b |
55 |
| 3 |
5-Cl |
C6H5 |
H |
4c |
91 |
| 4 |
6-Cl |
C6H5 |
H |
4d |
89 |
| 5 |
7-Cl |
C6H5 |
H |
4e |
79 |
| 6 |
5-F |
C6H5 |
H |
4f |
89 |
| 7 |
5-Br |
C6H5 |
H |
4g |
82 |
| 8 |
5-Me |
C6H5 |
H |
4h |
97 |
| 9 |
5-OMe |
C6H5 |
H |
4i |
99 |
| 10 |
5-NO2 |
C6H5 |
H |
4j |
84 |
| 11 |
H |
C6H5 |
Me |
4k |
99 |
| 12 |
H |
C6H5 |
Bn |
4l |
99 |
| 13 |
H |
C6H5 |
Allyl |
4m |
85 |
| 14d |
H |
4-BrC6H4 |
H |
4n |
99 |
| 15 |
H |
4-MeC6H4 |
H |
4o |
99 |
| 16 |
H |
Thienyl |
H |
4p |
76 |
| 17 |
H |
CH3 |
H |
4q |
96 |
Generally, all reactions proceeded smoothly to give the corresponding 3,3-disubstituted oxindoles in good to high yields (up to 99%). The effect of isatins with different-positioned halogens was first investigated. The 5-Cl, 6-Cl, and 7-Cl substituted isatins all showed good tolerance to this one-pot reaction and gave the products 4c–e in good to high yields (Table 2, entries 3–5). The 4-Cl substituted isatin, however, needed 2 equiv. of malononitrile and 50 °C reaction temperature to generate isatinylidene malononitrile, which then proceeded to decarboxylative Michael addition reaction to deliver the product 4b in moderated yield (55%, Table 2, entry 2). The steric hindrance of 4-Cl substituted isatin might be reducing the product yield. Isatins with other 5-halogen substituents (5-F and 5-Br), electron-donating groups (5-Me and 5-OMe), and electron-withdrawing groups (5-NO2) all reacted well with malononitrile and β-ketoacid 3a to afford the products 4f–j in high yields (Table 2, entries 6–10). The effect of different N-substituted isatins was also evaluated. N-Methyl, N-ethyl, and N-allyl substituted isatins all took part in the reaction and furnished the products 4k–m in excellent yields (Table 2, entries 11–13).
We then turn our attention to this one-pot reaction with isatin 1a, malononitrile, and different-substituted β-ketoacids 3b–e. It was found that the reactions proceeded smoothly to deliver the desired products 4n–q in good to excellent yields (Table 2, entries 14–17). In the case of 4-Br substituted β-ketoacid 3b, we discovered that it needed 3.6 equivalents of 3b to complete the reaction. It might be the deactivating property of halogens decreasing the nucleophilicity of 3b toward decarboxylative Michael addition reaction.
Having developed the above efficient one-pot sequential Knoevenagel condensation/decarboxylative Michael addition reaction of isatins, malononitrile and β-ketoacids, our next goal is to extend this reaction to malonic acid half thioesters (MAHTs). As shown in Table 3, to our delight, the one-pot reaction between isatin 1a, malononitrile 2 and MAHT 6a proceeded well in our conditions, affording the corresponding 5a in 99% yield (Table 3, entry 1). Isatins with different-positioned Cl atoms, 5-halogen substituents, electron-donating groups, and electron-withdrawing groups all equally worked smoothly (Table 3, entries 2–10). The reaction was also applicable to different N-substituted isatins and malonic acid half thioesters (Table 3, entries 11–15). To our surprise, the attempt on this one-pot reaction with malonic acid half ester 6d was unsuccessful. No any decarboxylative Michael addition product was detected in our optimized conditions (Table 3, entry 16).
Table 3 One-pot reaction of isatins, malononitrile and MAHTsa
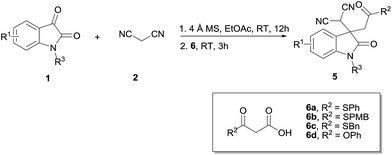
|
| Entry |
R1 |
R2 |
R3 |
5 |
Yieldb (%) |
| Unless otherwise noted, the reaction was carried out by using 0.1 mmol of 1a, 1.2 equiv. of 2, 30 mg 4 Å MS and 1.2 equiv. of 3 in 1 mL of solvent at RT for 12 h (step 1) and 3 h (step 2). Isolated yields. 2 equiv. of 2 at 50 °C for 18 h. No desired product was detected for step 2. |
| 1 |
H |
SPh |
H |
5a |
99 |
| 2c |
4-Cl |
SPh |
H |
5b |
68 |
| 3 |
5-Cl |
SPh |
H |
5c |
99 |
| 4 |
6-Cl |
SPh |
H |
5d |
99 |
| 5 |
7-Cl |
SPh |
H |
5e |
80 |
| 6 |
5-F |
SPh |
H |
5f |
98 |
| 7 |
5-Br |
SPh |
H |
5g |
90 |
| 8 |
5-Me |
SPh |
H |
5h |
95 |
| 9 |
5-OMe |
SPh |
H |
5i |
78 |
| 10 |
5-NO2 |
SPh |
H |
5j |
77 |
| 11 |
H |
SPh |
Me |
5k |
97 |
| 12 |
H |
SPh |
Bn |
5l |
88 |
| 13 |
H |
SPh |
Allyl |
5m |
91 |
| 14 |
H |
SPMB |
H |
5n |
86 |
| 15 |
H |
SBn |
H |
5o |
92 |
| 16d |
H |
OPh |
H |
— |
— |
The structure of the products was also confirmed based on the X-ray crystallographic analysis.16 Taking advantage of the crystallographic structure of 4g and 5g, all other product structures were deduced by referring to that (Fig. 2).
 |
| | Fig. 2 X-ray crystal structures of 4g and 5g. | |
To gain an insight into the role of 4 Å molecular sieves in the Knoevenagel condensation step, two control experiments were performed. As shown in Scheme 1, the isatylidene malononitrile 7 could be prepared from isatin 1a and malononitrile 2 using EA as a solvent at room temperature for 12 h without any additives (monitored by TLC) (Scheme 1a). To our delight, the reaction time was reduced to 3 h when 4 Å MS were added as an additive (Scheme 1b). From these data, we realized the 4 Å MS not only worked at the decarboxylative Michael addition reaction step, but also promoted the reaction rate of Knoevenagel condensation step. However, due to the different electronic nature of isatins, we set up the reaction time to 12 h for Knoevenagel condensation step to insure the completion of reaction.
 |
| | Scheme 1 Control experiments. | |
We then turn our attention to the practicality of our method by employing the reaction in preparative scale and the successful recycling of the molecular sieves. As illustrated in Scheme 2, the recovered molecular sieves were able to catalyse the reaction for up to three runs in preparative scale without any decrease in reactivity.
 |
| | Scheme 2 Preparative scales experiments and results with recycled molecular sieves. Reaction conditions: 147 mg (1 mmol) of 1a, 79 mg (1.2 mmol) of 2, 300 mg 4 Å MS and 235 mg (1.2 mmol) of 6a in 10 mL of EA at RT for 12 h (step 1) and 3 h (step 2). | |
Finally, the asymmetric feasibility of this one-pot reaction of isatin 1a, malononitrile 2, and MAHT 6a was investigated. As shown in Table 4, various cinchona alkaloid catalysts 8a–e were screened for this reaction, however, the product 5a was furnished in low enantioselectivities (<20% ee) (Table 4, entries 1–5). The N-protected substrate 1l was also examined under different reaction temperatures using 8d as a catalyst. The results showed almost no enantioselectivity was observed (Table 4, entries 6 and 7). These results indicated that the chirality of the products was difficult to control under the current reaction conditions.
Table 4 Conditions optimizationa
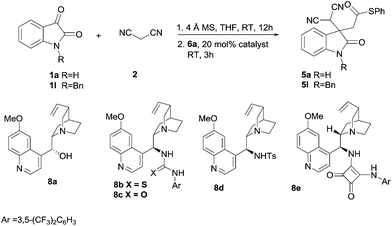
|
| Entry |
1 |
Catalyst |
Solvent |
Yieldb (%) |
eec (%) |
| Unless otherwise noted, the reaction was carried out by using 0.1 mmol of 1a, 1.2 equiv. of 2, 30 mg 4 Å MS, 20 mol% catalyst and 1.2 equiv. of 3 in 1 mL of THF at RT for 12 h (step 1) and 3 h (step 2). Isolated yields. Determinated by chiral HPLC analysis. At −20 °C. |
| 1 |
1a |
8a |
THF |
90 |
4 |
| 2 |
1a |
8b |
THF |
47 |
0 |
| 3 |
1a |
8c |
THF |
95 |
10 |
| 4 |
1a |
8d |
THF |
93 |
20 |
| 5 |
1a |
8e |
THF |
88 |
14 |
| 6 |
1l |
8d |
THF |
98 |
0 |
| 7d |
1l |
8d |
THF |
76 |
3 |
Conclusions
In conclusion, we have successfully developed a one-pot, molecular sieve mediated sequential Knoevenagel condensation/decarboxylative Michael addition reaction of β-ketoacids and MAHTs with isatylidene malononitriles, which are generated in situ from commercially available isatins and malononitrile. The described synthetic protocols were highly efficient, and were also broad in substrate scope, affording 3,3-disubstituted oxindoles with an all carbon quaternary center in high yields. Notably, we had shown the above reactions were carried out under very mild, base-free conditions, and molecular sieves were the sole activator for both reactions.
Experimental
General experimental details
All commercially available reagents were used without further purification unless otherwise stated. All reaction solvents were used as received. Proton nuclear magnetic resonance (1H NMR) spectra were recorded on a commercial instrument at 400 MHz. Carbon-13 nuclear magnetic resonance (13C NMR) spectra were recorded at 100 MHz. The proton signal for residual non-deuterated solvent (δ 7.26 for CHCl3) was used as an internal reference for 1H NMR spectra. For 13C NMR spectra, chemical shifts are reported relative to the δ 77.0 resonance of CHCl3. Coupling constants are reported in Hz. Infrared (IR) spectra were recorded on a commercial FTIR instrument. Melting points were determined on a BUCHI B-545 melting point apparatus and are un-corrected. High resolution mass spectra were recorded on a commercial high resolution mass spectrometer. The TLC plates were visualized with UV light and/or by staining with Hanessian solution (ceric sulfate and ammonium molybdate in aqueous sulfuric acid). Column chromatography was generally performed using Kieselgel 60 (230–400 mesh) silica gel, typically using a 50–100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio of silica gel to crude product. β-keto acids 3 and Malonic acid half thioesters 6 were prepared according to the literature procedure.13,17
1 weight ratio of silica gel to crude product. β-keto acids 3 and Malonic acid half thioesters 6 were prepared according to the literature procedure.13,17
General procedure for the synthesis of 4
To a solution of isatins 1 (0.1 mmol), malononitrile 2 (0.12 mmol) and MS 4 Å (30 mg) in EA (1 mL) was stirred at room temperature (28–30 °C) for 12 h. β-Keto acids 3 (0.12 mmol) was added and the reaction mixture was stirred at room temperature for 3 h. After completion of the reaction, the mixture was concentrated in vacuum and the crude was purified by silica gel flash chromatography (hexanes/EA 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the pure products 4.
1) to afford the pure products 4.
2-(2-Oxo-3-(2-oxo-2-phenylethyl)indolin-3-yl)malononitrile (4a). White powder; yield: 94%; mp: 116–117 °C; IR (neat): 3672, 3232, 2988, 2970, 2901, 1718, 1684, 1619, 1597, 1580 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.77 (bs, 1H), 7.86–7.87 (m, 2H), 7.58 (t, J = 7.6 Hz, 1H), 7.42–7.47 (m, 3H), 7.31 (td, J = 7.8, 1.1 Hz, 1H), 7.01–7.09 (m, 2H), 4.72 (s, 1H), 4.12 (d, J = 17.9 Hz, 1H), 3.78 (d, J = 17.9 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 194.6, 175.9, 141.6, 135.3, 134.3, 130.8, 128.9, 128.2, 125.8, 123.8, 123.6, 111.3, 110.6, 109.7, 49.7, 42.2, 30.4; HRMS (ESI): calcd for C19H13N3O2Na [M + Na]+: 338.0905; found: 338.0901.
2-(4-Chloro-2-oxo-3-(2-oxo-2-phenylethyl)indolin-3-yl) malononitrile (4b). White powder; yield: 55%; mp: 206–207 °C; IR (neat): 3250, 2907, 2874, 1716, 1706, 1685, 1618, cm−1; 1H NMR (400 MHz, CD3OD) δ 7.93–7.91 (m, 2H), 7.64–7.60 (m, 1H), 7.50–7.47 (m, 2H), 7.33 (t, J = 8.1 Hz, 1H), 7.00–6.97 (m, 2H), 4.66 (d, J = 17.8 Hz, 1H), 3.86 (d, J = 17.8 Hz, 1H); 13C NMR (100 MHz, CD3OD) δ 194.5, 175.4, 145.6, 135.3, 133.7, 131.6, 129.8, 128.6, 127.7, 122.9, 122.9, 110.0, 109.9, 109.2, 50.3, 40.6; HRMS (ESI): calcd for C19H11N3O235ClNa [M + Na]+: 372.0516; found: 372.0515.
2-(5-Chloro-2-oxo-3-(2-oxo-2-phenylethyl)indolin-3-yl) malononitrile (4c). White powder; yield: 91%; mp: 112–113 °C; IR (neat): 3672, 3232, 2988, 2970, 2901, 1723, 1683, 1619, 1597, 1581 cm−1; 1H NMR (400 MHz, CDCl3) δ 9.17 (bs, 1H), 7.85–7.86 (m, 2H), 7.60 (t, J = 7.3 Hz, 1H), 7.47–7.43 (m, 3H), 7.29–7.26 (m, 1H), 6.95 (d, J = 8.4 Hz, 1H), 4.70 (d, J = 2.7 Hz, 1H), 4.11 (d, J = 18.0 Hz, 1H), 3.80 (dd, J = 18.0, 2.7 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 194.4, 175.6, 140.3, 140.2, 135.0, 134.5, 130.9, 129.0, 128.9, 128.3, 127.4, 124.3, 112.4, 110.3, 109.5, 49.9, 42.3, 30.3; HRMS (ESI): calcd for C19H11N3O235Cl [M − H]−: 348.0546; found: 348.0540.
2-(6-Chloro-2-oxo-3-(2-oxo-2-phenylethyl)indolin-3-yl) malononitrile (4d). Yellow powder; yield: 89%; mp: 197–198 °C; IR (neat): 3672, 3267, 2989, 2972, 2902, 1716, 1687, 1616, 1596, 1582 cm−1; 1H NMR (400 MHz, d-acetone) δ 10.3 (bs, 1H), 7.99–7.97 (m, 2H), 7.67–7.62 (m, 1H), 7.55–7.49 (m, 3H), 7.15 (m, 1H), 7.09 (dd, J = 8.4, 2.0 Hz, 1H), 5.24 (s, 1H), 4.38 (d, J = 18.2 Hz, 1H), 4.05 (d, J = 18.2 Hz, 1H); 13C NMR (100 MHz, d-acetone) δ 194.5, 175.3, 144.8, 135.7, 135.3, 133.9, 128.8, 128.1, 125.7, 125.1, 122.1, 111.2, 110.7, 110.6, 49.0, 42.3, 30.5; HRMS (ESI): calcd for C19H12N3O235ClNa [M + Na]+: 372.0516; found: 3372.0518.
2-(7-Chloro-2-oxo-3-(2-oxo-2-phenylethyl)indolin-3-yl) malononitrile (4e). Orange solid; yield: 79%; mp: 110–111 °C; IR (neat): 3662, 2988, 2972, 2902, 1725, 1684, 1621, 1596, 1580 cm−1; 1H NMR (400 MHz, d-acetone) δ 10.5 (bs, 1H), 8.00–7.97 (m, 2H), 7.67–7.62 (m, 1H), 7.53–7.49 (m, 3H), 7.42 (dd, J = 8.3, 1.0 Hz, 1H), 7.09 (t, J = 8.0, 1H), 5.26 (s, 1H), 4.39 (d, J = 18.2 Hz, 1H), 4.07 (dd, J = 18.2 Hz, 1H); 13C NMR (100 MHz, d-acetone) δ 194.4, 175.0, 141.2, 135.7, 133.9, 130.2, 128.8, 128.6, 128.1, 123.5, 122.2, 115.1, 111.1, 110.6, 50.1, 42.5, 30.6; HRMS (ESI): calcd for C19H12N3O235ClNa [M + Na]+: 372.0516; found: 372.0519.
2-(5-Fluoro-2-oxo-3-(2-oxo-2-phenylethyl)indolin-3-yl) malononitrile (4f). White powder; yield: 89%; mp: 114–115 °C; IR (neat): 3672, 3232, 2988, 2970, 2902, 1723, 1683, 1632, 1597, 1581 cm−1; 1H NMR (400 MHz, CDCl3) δ 9.23 (bs, 1H), 7.88–7.86 (m, 2H), 7.60 (t, J = 7.2 Hz, 1H), 7.43–7.47 (m, 2H), 7.22 (dd, J = 7.6, 2.4 Hz, 1H), 6.95–7.03 (m, 2H), 4.74 (s, 1H), 4.10 (d, J = 18.0 Hz, 1H), 3.80 (dd, J = 18.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 194.6, 176.0, 159.2 (d, J = 246 Hz), 137.7 (d, J = 2.3 Hz), 135.1, 134.5, 129.0, 128.3, 127.1 (d, J = 7.8 Hz), 117.4 (d, J = 24.3 Hz), 112.3 (d, J = 1.5 Hz), 112.1 (d, J = 15.5 Hz), 110.4, 109.5, 50.1, 42.2, 30.2; HRMS (ESI): calcd for C19H12N3O2FNa [M + Na]+: 356.0811; found: 356.0804.
2-(5-Bromo-2-oxo-3-(2-oxo-2-phenylethyl)indolin-3-yl) malononitrile (4g). White powder; yield: 82%; mp: 114–115 °C; IR (neat): 3672, 3232, 2988, 2973, 2901, 1724, 1682, 1616, 1596, 1580 cm−1; 1H NMR (400 MHz, CDCl3) δ 9.16 (bs, 1H), 7.87–7.86 (m, 2H), 7.63–7.57 (m, 2H), 7.47–7.41 (m, 3H), 6.90 (d, J = 8.4 Hz, 1H), 4.68 (s, 1H), 4.10 (d, J = 18.0 Hz, 1H), 3.80 (d, J = 18.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 194.4, 175.5, 140.7, 135.0, 134.6, 133.8, 129.0, 128.3, 127.8, 127.0, 116.2, 112.8, 110.3, 109.4, 49.8, 42.4, 30.3; HRMS (ESI): calcd for C19H12N3O279BrNa [M + Na]+: 416.0011; found: 416.0015.
2-(5-Methyl-2-oxo-3-(2-oxo-2-phenylethyl)indolin-3-yl) malononitrile (4h). White powder; yield: 97%; mp: 121–122 °C; IR (neat): 3672, 3232, 2988, 2970, 2902, 1717, 1688, 1625, 1597, 1581 cm−1; 1H NMR (400 MHz, CDCl3) δ 9.09 (bs, 1H), 7.89–7.87 (m, 2H), 7.58 (t, J = 7.6 Hz, 1H), 7.44 (t, J = 7.6 Hz, 1H), 7.26 (s, 1H), 7.09 (d, J = 8.0 Hz, 1H), 6.91 (d, J = 8.0 Hz, 1H), 4.73 (s, 1H), 4.10 (d, J = 17.8 Hz, 1H), 3.78 (d, J = 17.8 Hz, 1H), 2.28 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 194.7, 176.0, 139.1, 135.4, 134.2, 133.2, 131.2, 128.9, 128.3, 125.8, 124.4, 111.1, 110.7, 109.8, 49.8, 42.2, 30.5, 21.2; HRMS (ESI): calcd for C20H15N3O2Na [M + Na]+: 352.1062; found: 352.1056.
2-(5-Methoxy-2-oxo-3-(2-oxo-2-phenylethyl)indolin-3-yl) malononitrile (4i). White powder; yield: 99%; mp: 132–133 °C; IR (neat): 3672, 3232, 2988, 2971, 2902, 1717, 1688, 1598 cm−1; 1H NMR (400 MHz, CDCl3) δ 9.09 (bs, 1H), 7.88–7.86 (m, 2H), 7.58 (t, J = 7.4 Hz, 1H), 7.43 (t, J = 7.8 Hz, 1H), 7.06 (d, J = 2.5 Hz, 1H), 6.94 (d, J = 8.5 Hz, 1H), 6.82 (dd, J = 8.5, 2.5 Hz, 1H), 4.75 (s, 1H), 4.08 (d, J = 18.0 Hz, 1H), 3.77 (d, J = 18.0 Hz, 1H), 3.73 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 194.6, 175.8, 156.3, 135.3, 134.8, 134.3, 128.9, 128.2, 127.0, 115.1, 111.7, 111.1, 110.6, 109.8, 55.8, 50.1, 42.1, 30.4; HRMS (ESI): calcd for C20H15N3O3Na [M + Na]+: 368.1011; found: 368.1007.
2-(5-Nitro-2-oxo-3-(2-oxo-2-phenylethyl)indolin-3-yl) malononitrile (4j). Yellow powder; yield: 84%; mp: 187–188 °C; IR (neat): 3664, 3237, 2988, 2973, 2901, 1727, 1716, 1686, 1624, 1605 cm−1; 1H NMR (400 MHz, d-acetone) δ 10.8 (bs, 1H), 8.51 (d, J = 2.3 Hz, 1H), 8.36 (dd, J = 8.8, 2.3 Hz, 1H), 8.01–7.98 (m, 2H), 7.64 (td, J = 7.4, 1.3 Hz, 1H), 7.53–7.49 (m, 2H), 7.36 (d, J = 8.6 Hz, 1H), 5.39 (s, 1H), 4.65 (d, J = 18.2 Hz, 1H), 4.18 (d, J = 18.2 Hz, 1H); 13C NMR (100 MHz, d-acetone) δ 194.6, 175.6, 149.6, 143.2, 135.5, 134.0, 128.8, 128.2, 127.9, 127.1, 120.0, 110.9, 110.4, 110.4, 49.3, 42.6, 30.3; HRMS (ESI): calcd for C19H11N4O4 [M − H]−: 359.0780; found: 359.0774.
2-(1-Methyl-2-oxo-3-(2-oxo-2-phenylethyl)indolin-3-yl)malononitrile (4K). White powder; yield: 99%; mp: 153–154 °C; IR (neat): 3672, 2989, 2969, 2914, 1712, 1694, 1615, 1597 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.87–7.84 (m, 2H), 7.58 (t, J = 7.4 Hz, 1H), 7.49–7.42 (m, 4H), 7.12 (td, J = 7.6, 0.8 Hz, 1H), 7.02 (d, J = 7.9 Hz, 1H), 4.63 (s, 1H), 4.10 (d, J = 17.6 Hz, 1H), 3.71 (d, J = 17.6 Hz, 1H), 3.67 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 194.1, 173.9, 144.5, 135.4, 134.2, 130.8, 128.9, 128.1, 125.2, 123.6, 123.5, 110.6, 109.5, 109.4, 49.3, 42.2, 30.6, 27.0; HRMS (ESI): calcd for C20H15N3O2Na [M + Na]+: 352.1062; found: 352.1060.
2-(1-Benzyl-2-oxo-3-(2-oxo-2-phenylethyl)indolin-3-yl)malononitrile (4l). Red solid; yield: 99%; mp: 120–121 °C; IR (neat): 3671, 2989, 2972, 2902, 1713, 1687, 1612, 1597 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.90–7.88 (m, 2H), 7.59 (t, J = 7.5 Hz, 1H), 7.51–7.28 (m, 9H), 7.08 (td, J = 7.6, 0.8 Hz, 1H), 6.86 (d, J = 8.0 Hz, 1H), 5.07 (s, 2H), 4.70 (s, 1H), 4.16 (d, J = 17.7 Hz, 1H), 3.76 (d, J = 17.7 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 194.0, 174.2, 143.8, 135.4, 134.8, 134.2, 130.7, 128.9, 128.8, 128.2, 127.9, 127.5, 125.3, 123.7, 123.6, 110.7, 110.6, 109.7, 49.4, 44.9, 42.4, 30.7; HRMS (ESI): calcd for C26H19N3O2Na [M + Na]+: 428.1375; found: 428.1376.
2-(1-Allyl-2-oxo-3-(2-oxo-2-phenylethyl)indolin-3-yl)malononitrile (4m). Colorless oil; yield: 85%; IR (neat): 3672, 2923, 2845, 1714, 1688, 1612 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.87–7.85 (m, 2H), 7.60–7.56 (m, 1H), 7.50–7.38 (m, 4H), 7.11 (td, J = 7.7, 1.0 Hz, 1H), 7.02 (d, J = 7.9 Hz, 1H), 5.99–5.89 (m, 1H), 5.49 (dd, J = 17.2, 1.0 Hz, 1H), 5.33 (dd, J = 10.3, 1.0 Hz, 1H), 4.63 (s, 1H), 4.56–4.42 (m, 2H), 4.12 (d, J = 17.8 Hz, 1H), 3.72 (d, J = 17.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 194.0, 173.7, 143.8, 135.4, 134.2, 130.7, 130.7, 128.9, 128.1, 125.2, 123.6, 123.5, 118.6, 110.7, 110.4, 109.6, 49.2, 43.3, 42.4, 30.7; HRMS (ESI): calcd for C22H17N3O2Na [M + Na]+: 378.1218; found: 378.1212.
2-(3-(2-(4-Bromophenyl)-2-oxoethyl)-2-oxoindolin-3-yl)malononitrile (4n). White powder; yield: 99%; mp: 205–206 °C; IR (neat): 3271, 2923, 2866, 2845, 1716, 1683, 1617, 1586 cm−1; 1H NMR (400 MHz, d-acetone) δ 10.13 (bs, 1H), 7.92 (d, J = 8.7 Hz, 2H), 7.70 (d, J = 8.7 Hz, 2H), 7.52 (d, J = 7.6 Hz, 1H), 7.35 (td, J = 7.8, 1.2 Hz, 1H), 7.11–7.03 (m, 2H), 5.21 (s, 1H), 4.33 (d, J = 18.0 Hz, 1H), 3.99 (d, J = 18.0 Hz, 1H); 13C NMR (100 MHz, d-acetone) δ 193.8, 175.2, 143.3, 132.0, 130.2, 130.0, 128.3, 126.7, 123.7, 122.3, 111.3, 110.6, 110.4, 49.2, 42.1, 30.6; HRMS (ESI): calcd for C19H12N3O279BrNa [M + Na]+: 416.0011; found: 416.0018.
2-(3-(2-(4-Methylphenyl)-2-oxoethyl)-2-oxoindolin-3-yl)malononitrile (4o). White powder; yield: 99%; mp: 175–176 °C; IR (neat): 3672, 3265, 2988, 2901, 1725, 1680, 1610 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.94 (bs, 1H), 7.77 (d, J = 8.4 Hz, 2H), 7.46 (d, J = 7.5 Hz, 1H), 7.31 (td, J = 7.7, 1.1 Hz, 1H), 7.23 (d, J = 8.4 Hz, 2H), 7.07 (td, J = 7.7, 0.9 Hz, 1H), 7.01 (d, J = 7.7 Hz, 1H), 4.72 (s, 1H), 4.09 (d, J = 17.7 Hz, 1H), 3.74 (d, J = 17.7 Hz, 1H), 2.39 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 194.2, 175.9, 145.4, 141.5, 132.9, 130.8, 129.6, 128.4, 125.8, 123.9, 123.5, 111.3, 110.6, 109.7, 49.7, 42.0, 30.4, 21.8; HRMS (ESI): calcd for C20H15N3O2Na [M + Na]+: 352.1062; found: 352.1065.
2-(2-Oxo-3-(2-oxo-2-(thiophen-2-yl)ethyl)indolin-3-yl)malononitrile (4p). White powder; yield: 76%; mp: 168–169 °C; IR (neat): 3681, 3255, 2973, 2923, 2866, 2845, 1726, 1693, 1659, 1618 cm−1; 1H NMR (400 MHz, CDCl3) δ 9.20 (bs, 1H), 7.74 (d, J = 3.8 Hz, 1H), 7.67 (d, J = 4.9 Hz, 1H), 7.47 (d, J = 7.4 Hz, 1H), 7.30 (t, J = 7.4 Hz, 1H), 7.11–7.00 (m, 3H), 4.79 (s, 1H), 4.00 (d, J = 17.5 Hz, 1H), 3.71 (d, J = 17.5 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 187.5, 175.7, 142.3, 141.6, 135.5, 133.5, 130.9, 128.7, 125.5, 123.9, 123.6, 111.4, 110.6, 109.8, 49.7, 42.2, 30.2; HRMS (ESI): calcd for C17H11N3O2SNa [M + Na]+: 344.0470; found: 344.0467.
2-(2-Oxo-3-(2-oxopropyl)indolin-3-yl)malononitrile (4q)6. Known compound; white powder; yield: 96%; 1H NMR (400 MHz, d-acetone) δ 10.0 (bs, 1H), 7.46 (d, J = 7.5 Hz, 1H), 7.34 (td, J = 7.7, 1.2 Hz, 1H), 7.09–7.03 (m, 2H), 5.10 (s, 1H), 3.67 (d, J = 18.1 Hz, 1H), 3.38 (d, J = 17.5 Hz, 1H), 2.12 (s, 3H).
General procedure for the synthesis of 5
To a solution of isatins 1 (0.1 mmol), malononitrile 2 (0.12 mmol) and MS 4 Å (30 mg) in EA (1 mL) was stirred at room temperature (28–30 °C) for 12 h. Malonic acid half thioesters 6 (0.12 mmol) was added and the reaction mixture was stirred at room temperature for 3 h. After completion of the reaction, the mixture was concentrated in vacuum and the crude was purified by silica gel flash chromatography (hexanes/EA 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the pure products 5.
1) to afford the pure products 5.
S-Phenyl 2-(3-(dicyanomethyl)-2-oxoindolin-3-yl)ethanethio-ate (5a). White powder; yield: 99%; mp: 73–74 °C; IR (neat): 3356, 2988, 2909, 1727, 1699 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.77 (s, 1H), 7.45 (d, J = 7.5 Hz, 1H), 7.21–7.18 (m, 2H), 7.12 (t, J = 7.6 Hz, 1H), 6.81 (d, J = 7.6 Hz, 1H), 4.52 (s, 1H), 3.50 (d, J = 16.2 Hz, 1H), 3.36 (d, J = 16.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 193.4, 174.7, 141.3, 134.5, 131.2, 130.3, 129.5, 129.6, 126.0, 124.5, 124.1, 123.8, 111.6, 110.3, 109.5, 50.2, 45.6, 30.1; HRMS (ESI): calcd for C19H13N3O2SNa [M + Na]+: 370.0626; found: 370.0626.
S-Phenyl-2-(4-chloro-3-(dicyanomethyl)-2-oxoindolin-3-yl)-ethanethioate (5b). Light brown powder; yield: 68%; mp: 166–167 °C; IR (neat): 3750, 3141, 2988, 2901, 1717, 1699 cm−1; 1H NMR (400 MHz, d6-DMSO) δ 10.1 (s, 1H), 6.98–6.91 (m, 3H), 6.78 (t, J = 7.9 Hz, 1H), 6.71–6.69 (m, 2H), 6.51 (d, J = 8.8 Hz, 1H), 6.29 (d, J = 7.2 Hz, 1H), 6.02 (s, 1H), 3.31 (d, J = 15.3 Hz, 1H), 2.88 (d, J = 15.3 Hz, 1H); 13C NMR (100 MHz, d6-DMSO) δ 192.6, 176.7, 144.5, 134.2, 131.3, 130.6, 129.7, 129.4, 126.8, 126.2, 122.3, 108.7, 74.0, 48.3; HRMS (ESI): calcd for C19H12N3O2SNa35Cl [M + Na]+: 404.0326; found: 404.0238.
S-Phenyl-2-(5-chloro-3-(dicyanomethyl)-2-oxoindolin-3-yl)-ethanethioate (5c). White powder; yield: 99%; mp 194–195 °C; IR (neat): 3750, 3335, 2971, 2901, 1724, 1619 cm−1; 1H NMR (400 MHz, d6-acetone) δ 10.2 (s, 1H), 7.64 (d, J = 2.2 Hz, 1H), 7.43–7.38 (m, 4H), 7.30–7.28 (m, 2H), 7.08 (d, J = 8.4 Hz, 1H), 5.26 (s, 1H), 3.86 (d, J = 16.5 Hz, 1H), 3.68 (d, J = 16.5 Hz, 1H); 13C NMR (100 MHz, d6-acetone) δ 192.2, 174.0, 141.9, 134.5, 130.6, 129.9, 129.4, 127.4, 127.2, 126.7, 124.5, 112.0, 110.9, 110.3, 49.8, 45.5, 29.9; HRMS (ESI): calcd for C19H12N3O2SNa35Cl [M + Na]+: 404.0326; found: 404.0232.
S-Phenyl-2-(6-chloro-3-(dicyanomethyl)-2-oxoindolin-3-yl)-ethanethioate (5d). Light brown powder; mp: 67–68 °C; IR (neat): 3750, 3335, 2988, 2901, 1734, 1670, 1615 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.84 (s, 1H), 7.47 (d, J = 8.1 Hz, 1H), 7.43–7.39 (m, 3H), 7.32–7.29 (m, 2H), 7.18 (dd, J = 8.1, 1.8 Hz, 1H), 6.86 (d, J = 1.8 Hz, 1H), 4.53 (s, 1H), 3.60 (d, J = 16.3 Hz, 1H), 3.46 (d, J = 16.3 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 193.5, 174.6, 142.5, 137.1, 134.5, 130.5, 129.7, 125.7, 125.0, 123.8, 122.8, 112.2, 110.1, 109.3, 49.9, 45.6, 30.1; HRMS (ESI): calcd for C19H12N3O2SNa35Cl [M + Na]+: 404.0326; found: 404.0235.
S-Phenyl-2-(7-chloro-3-(dicyanomethyl)-2-oxoindolin-3-yl)-ethanethioate (5e). Orange powder; yield: 80%; mp: 142–143 °C; IR (neat): 3750, 3287, 2988, 2908, 1721, 1702, 1619 cm−1; 1H NMR (400 MHz, d6-acetone) δ 10.4 (s, 1H), 7.56 (d, J = 7.5 Hz, 1H), 7.46–7.38 (m, 4H), 7.29–7.26 (m, 2H), 7.12 (t, J = 7.9 Hz, 1H), 5.24 (s, 1H), 3.81 (d, J = 16.4 Hz, 1H), 3.68 (d, J = 16.4 Hz, 1H); 13C NMR (100 MHz, d6-acetone) δ 193.0, 174.8, 141.6, 135.3, 131.6, 130.7, 130.3, 128.1, 127.6, 124.7, 123.6, 116.3, 111.7, 111.2, 51.4, 46.6, 31.0; HRMS (ESI): calcd for C19H11N3O2S35Cl [M − H]−: 380.0261; found: 380.0269.
S-Phenyl-2-(3-(dicyanomethyl)-5-fluoro-2-oxoindolin-3-yl)-ethanethioate (5f). Pink powder; yield: 98%; mp: 203–204 °C; IR (neat): 3750, 3337, 2973, 2922, 2845, 1728, 1697, 1624, 1607 cm−1; 1H NMR (400 MHz, d6-acetone) δ 10.1 (s, 1H), 7.45–7.38 (m, 4H), 7.27–7.30 (m, 2H), 7.16–7.21 (m, 1H), 7.07 (dd, J = 8.6, 4.3 Hz, 1H), 5.24 (s, 1H), 3.82 (d, J = 16.5 Hz, 1H), 3.66 (d, J = 16.5 Hz, 1H); 13C NMR (100 MHz, d6-acetone) δ 193.0, 175.0, 159.6 (d, J = 238 Hz), 140.0 (d, J = 2.3 Hz), 135.3, 130.7, 130.2, 127.8 (d, J = 8.4 Hz), 127.6, 117.9 (d, J = 23.7 Hz), 112.9 (d, J = 25.3 Hz), 112.5 (d, J = 8.3 Hz), 111.7, 111.2, 50.9 (d, J = 1.4 Hz), 46.4, 30.8; HRMS (ESI): calcd for C19H12N3O2SNaF [M + Na]+: 388.0532; found: 388.0527.
S-Phenyl-2-(5-bromo-3-(dicyanomethyl)-2-oxoindolin-3-yl)-ethanethioate (5g). White powder; yield: 90%; mp: 172–173 °C; IR (neat): 3750, 3671, 2973, 2920, 2844, 1734, 1670, 1616 cm−1; 1H NMR (400 MHz, d6-acetone) δ 10.2 (s, 1H), 7.77 (d, J = 2.0 Hz, 1H), 7.57 (dd, J = 8.4, 2.0 Hz, 1H), 7.46–7.39 (m, 3H), 7.30–7.28 (m, 2H), 7.03 (d, J = 8.4 Hz, 1H), 5.26 (s, 1H), 3.86 (d, J = 16.5 Hz, 1H), 3.67 (d, J = 16.5 Hz, 1H); 13C NMR (100 MHz, d6-acetone) δ 193.1, 174.6, 143.1, 135.3, 134.3, 130.7, 130.2, 128.6, 128.1, 127.5, 115.2, 113.3, 111.7, 111.1, 50.5, 46.3, 30.7; HRMS (ESI): calcd for C19H13N3O2SNa79Br [M + Na]+: 447.9731; found: 447.9733.
S-Phenyl-2-(3-(dicyanomethyl)-5-methyl-2-oxoindolin-3-yl)-ethanethioate (5h). White powder; yield: 95%; mp: 180–181 °C; IR (neat): 3750, 3671, 3327, 2973, 2923, 1726, 1702, 1623 cm−1; 1H NMR (400 MHz, d6-acetone) δ 9.99 (s, 1H), 7.43–7.37 (m, 4H), 7.27–7.25 (m, 2H), 7.20 (dt, J = 7.9, 0.8 Hz, 1H), 6.94 (d, J = 8.0 Hz, 1H), 5.18 (s, 1H), 3.71 (d, J = 16.1 Hz, 1H), 3.59 (d, J = 16.1 Hz, 1H), 2.35 (s, 3H); 13C NMR (100 MHz, d6-acetone) δ 192.8, 175.0, 141.2, 135.2, 132.8, 131.7, 130.5, 130.1, 127.6, 126.2, 125.4, 112.0, 111.3, 111.2, 50.5, 46.4, 30.9, 21.1; HRMS (ESI): calcd for C20H15N3O2SNa [M + Na]+: 384.0783; found: 384.0774.
S-Phenyl-2-(3-(dicyanomethyl)-5-methoxy-2-oxoindolin-3-yl)-ethanethioate (5i). White powder; yield: 78%; mp: 141–142 °C; IR (neat): 3341, 2973, 2923, 1709, 1683, 1610 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.76 (s, 1H), 7.40–7.34 (m, 3H), 7.27 (dd, J = 7.8, 1.6 Hz, 2H), 7.08 (d, J = 2.4 Hz, 1H), 6.85 (dd, J = 8.6, 2.5 Hz, 1H), 6.79 (d, J = 8.6 Hz, 1H), 4.58 (s, 1H), 3.80 (s, 3H), 3.52 (d, J = 16.3 Hz, 1H), 3.69 (d, J = 16.3 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 193.4, 174.5, 156.5, 134.5, 134.4, 130.2, 129.6, 126.1, 125.7, 115.8, 112.2, 111.0, 110.3, 109.5, 50.6, 56.0, 45.5, 30.1; HRMS (ESI): calcd for C20H15N3O3SNa [M + Na]+: 400.0732; found: 400.0723.
S-Phenyl-2-(3-(dicyanomethyl)-5-nitro-2-oxoindolin-3-yl)-ethanethioate (5j). Light yellow powder; yield: 77%; mp: 187–188 °C; IR (neat): 3681, 2973, 2923, 2867, 2845, 1735, 1698, 1604 cm−1; 1H NMR (400 MHz, d6-acetone) δ 9.74 (s, 1H), 7.56 (d, J = 2.3 Hz, 1H), 7.38 (dd, J = 8.7, 2.3 Hz, 1H), 6.46–6.38 (m, 3H), 6.32–6.28 (m, 3H), 4.41 (s, 1H), 3.08 (d, J = 16.7 Hz, 1H), 2.81 (d, J = 16.7 Hz, 1H); 13C NMR (100 MHz, d6-acetone) δ 193.3, 145.3, 149.8, 144.0, 135.2, 130.6, 130.1, 128.2, 127.2, 127.2, 121.0, 111.5, 111.3, 110.8, 50.2, 46.2, 30.5; calcd for C19H11N4O4S [M − H]−: 391.0501; found: 391.0504.
S-Phenyl-2-(3-(dicyanomethyl)-1-methyl-2-oxoindolin-3-yl)ethanethioate (5k). White powder; yield: 97%; mp: 142–143 °C; IR (neat): 3672, 2973, 2893, 1717, 1685, 1613 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.54 (d, J = 7.5 Hz, 1H), 7.46 (t, J = 7.8 Hz, 1H), 7.35–7.41 (m, 3H), 7.25–7.28 (m, 2H), 7.20 (t, J = 7.6 Hz, 1H), 6.96 (d, J = 7.8 Hz, 1H), 4.55 (s, 1H), 3.53 (d, J = 16.1 Hz, 1H), 3.37 (d, J = 16.1 Hz, 1H), 3.29 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 192.6, 172.7, 144.2, 134.5, 131.2, 130.2, 129.5, 126.1, 124.1, 123.9, 110.4, 109.7, 109.4, 49.9, 45.5, 30.3, 27.1; HRMS (ESI): calcd for C20H15N3O2SNa [M + Na]+: 384.0783; found: 384.0779.
S-Phenyl-2-(1-benzyl-3-(dicyanomethyl)-2-oxoindolin-3-yl)-ethanethioate (5l). White powder; yield: 88%; mp: 106–107 °C; IR (neat): 3672, 2973, 2902, 1721, 1686, 1611 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.56 (d, J = 7.5 Hz, 1H), 7.45–7.26 (m, 11H), 7.17 (t, J = 7.6 Hz, 1H), 6.81 (d, J = 7.9 Hz, 1H), 5.06 (d, J = 15.8 Hz, 1H), 4.94 (d, J = 15.8 Hz, 1H), 4.65 (s, 1H), 3.56 (d, J = 16.0 Hz, 1H), 3.40 (d, J = 16.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 192.6, 173.0, 143.3, 134.6, 134.5, 131.1, 130.1, 129.5, 129.0, 128.0, 127.5, 126.2, 124.2, 124.0, 123.9, 110.8, 110.5, 109.5, 50.0, 45.8, 44.9, 30.1; HRMS (ESI): calcd for C26H19N3O2SNa [M + Na]+: 460.1062; found: 460.1070.
S-Phenyl-2-(1-allyl-3-(dicyanomethyl)-2-oxoindolin-3-yl)-ethanethioate (5m). Light brown powder; yield: 91%; mp: 135–136 °C; IR (neat): 3672, 2973, 2923, 2900, 1717, 1686, 1610 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.55 (d, J = 7.4 Hz, 1H), 7.43–7.35 (m, 4H), 7.27–7.25 (m, 2H), 7.19 (td, J = 7.6, 0.6 Hz, 1H), 6.96 (d, J = 7.9 Hz, 1H), 5.89–5.79 (m, 1H), 5.36 (d, J = 17.2 Hz, 1H), 5.26 (d, J = 10.5 Hz, 1H), 4.59 (s, 1H), 4.54–4.28 (m, 2H), 3.53 (d, J = 16.0 Hz, 1H), 3.36 (d, J = 16.0 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 192.5, 172.6, 143.4, 134.5, 131.1, 130.5, 130.1, 129.5, 126.2, 124.1, 123.9, 123.8, 118.7, 110.6, 110.5, 109.4, 49.8, 45.7, 43.3, 30.2; HRMS (ESI): calcd for C22H16N3O2SNa [M + Na]+: 386.0963; found: 386.0957.
S-(4-Methoxyphenyl)-2-(3-(dicyanomethyl)-2-oxoindolin-3-yl)-ethanethioate (5n). White powder; yield: 86%; mp: 178–179 °C; IR (neat): 3371, 2974, 2889, 1736, 1682, 1621 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.43 (s, 1H), 7.49 (d, J = 7.5 Hz, 1H), 7.35 (td, J = 7.8, 1.0 Hz, 1H), 7.18–7.14 (m, 3H), 6.90–6.87 (m, 3H), 4.55 (s, 1H), 3.77 (s, 3H), 3.52 (d, J = 16.2 Hz, 1H), 3.35 (d, J = 16.2 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 194.3, 174.4, 161.2, 141.2, 136.2, 131.2, 124.6, 124.2, 123.8, 116.6, 115.2, 111.5, 110.3, 109.4, 55.5, 50.3, 45.4, 30.1; HRMS (ESI): calcd for C20H15N3O3SNa [M + Na]+: 400.0732; found: 400.0730.
S-Benzyl-2-(3-(dicyanomethyl)-2-oxoindolin-3-yl)-ethanethioate (5o). White powder; yield: 92%; mp: 169–170 °C; IR (neat): 3672, 3363, 2973, 2902, 2874, 1711, 1668, 1624 cm−1; 1H NMR (400 MHz, CDCl3) δ 8.92 (s, 1H), 7.42–7.34 (m, 2H), 7.26–7.22 (m, 3H), 7.13–7.07 (m, 3H), 7.00 (d, J = 7.8 Hz, 1H), 4.60 (s, 1H), 4.03 (s, 2H), 3.44 (d, J = 15.8 Hz, 1H), 3.31 (d, J = 15.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 193.9, 174.9, 141.2, 136.3, 131.2, 128.8, 128.8, 127.6, 124.5, 124.2, 123.9, 111.5, 110.4, 109.6, 50.4, 45.7, 33.7, 30.1; HRMS (ESI): calcd for C20H15N3O2SNa [M + Na]+: 384.0783; found: 384.0781.
Acknowledgements
We are grateful for financial support from Ministry of Science and Technology, Taiwan (104-2113-M-033-009-MY2). We thank the mass center of institute of chemistry, Academia Sinica for HRMS measurement. We also acknowledge Prof. Chi-Wi Ong provides his helpful suggestion for preparing this manuscript.
Notes and references
- J. J. Badillo, N. V. Hanhan and A. K. Franz, Curr. Opin. Drug Discovery Dev., 2010, 13, 758 CAS.
-
(a) A. Canas-Rodriguez and P. R. Leeming, J. Med. Chem., 1972, 15, 762 CrossRef CAS PubMed;
(b) M. K. Uddin, S. G. Reignier, T. Coulter, C. Montalbetti, C. Grånäs, S. Butcher, C. KrogJensen and J. Felding, Bioorg. Med. Chem. Lett., 2007, 17, 2854 CrossRef CAS PubMed.
- S. W. Tam and R. Zaczek, Adv. Exp. Med. Biol., 1995, 363, 47 CrossRef CAS PubMed.
-
(a) B. Volk, J. Barkóczy, E. Hegedus, S. Udvari, I. Gacsályi, T. Mezei, K. Pallagi, H. Kompagne, G. Lévay, A. Egyed, L. G. Hársing, M. Spedding and G. Simig, J. Med. Chem., 2008, 51, 2522 CrossRef CAS PubMed;
(b) B. Volk, I. Gacsályi, K. Pallagi, L. Poszávácz, I. Gyönös, É. Szabó, T. Baký, M. Spedding, G. Simig and G. Szénási, J. Med. Chem., 2011, 54, 6657 CrossRef CAS PubMed.
- For reviews, see:
(a) C. V. Galliford and K. A. Scheidt, Angew. Chem., Int. Ed., 2007, 46, 8748 CrossRef CAS PubMed;
(b) C. Marti and E. M. Carreira, Eur. J. Org. Chem., 2003, 2209 CrossRef CAS;
(c) R. M. Williams and R. J. Cox, Acc. Chem. Res., 2003, 36, 127 CrossRef CAS PubMed;
(d) F. Zhou, Y.-L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381 CrossRef CAS;
(e) K. Shen, X.-H. Liu, L.-L. Lin and X.-M. Feng, Chem. Sci., 2012, 3, 327 RSC;
(f) R. Dalpozzo, G. Bartoli and G. Bencivenni, Chem. Soc. Rev., 2012, 41, 7247 RSC;
(g) G. S. Singh and Z. Y. Desta, Chem. Rev., 2012, 112, 6104 CrossRef CAS PubMed;
(h) J. Cheng, Y. Ishihara, B. Tan and C. F. Barbas III, ACS Catal., 2014, 4, 743 CrossRef.
-
(a) L. Liu, D. Wu, X. Li, S. Wang, H. Li, J. Li and W. Wang, Chem. Commun., 2012, 48, 1692 RSC;
(b) H. Zhao, Y.-B. Lan, Z.-M. Liu, Y. Wang, X.-W. Wang and J.-C. Tao, Eur. J. Org. Chem., 2012, 1935 CrossRef CAS;
(c) A. Kumar and S. S. Chimni, Beilstein J. Org. Chem., 2014, 10, 929 CrossRef PubMed.
-
(a) Y.-B. Lan, H. Zhao, Z.-M. Liu, G.-G. Liu, J.-C. Tao and X.-W. Wang, Org. Lett., 2011, 13, 4866 CrossRef CAS PubMed;
(b) D. B. Ramachary, C. Venkaiah and R. Madhavachary, Org. Lett., 2013, 15, 3042 CrossRef CAS PubMed.
-
(a) W.-B. Chen, Z.-J. Wu, Q.-L. Pei, L.-F. Cun, X.-M. Zhang and W.-C. Yuan, Org. Lett., 2010, 12, 3132 CrossRef CAS PubMed;
(b) X. Jiang, Y. Sun, J. Yao, Y. Cao, M. Kai, N. He, X. Zhang, Y. Wang and R. Wang, Adv. Synth. Catal., 2012, 354, 917 CrossRef CAS;
(c) F.-F. Pan, W. Yu, Z.-H. Qi, C. Qiao and X.-W. Wang, Synthesis, 2014, 46, 1143 CrossRef;
(d) H.-W. Zhao, B. Li, T. Tian, W. Meng, Z. Yang, X.-Q. Song, X.-Q. Chen and H.-L. Pang, Eur. J. Org. Chem., 2015, 3320 CrossRef CAS.
- Z.-M. Liu, N.-K. Li, X.-F. Huang, B. Wu, N. Li, C.-Y. Kwok, Y. Wang and X.-W. Wang, Tetrahedron, 2014, 70, 2406 CrossRef CAS; Z.-H. Qi, Y. Zhang, G.-Y. Ruan, Y. Zhang, Y. Wang and X.-W. Wang, RSC Adv., 2015, 5, 34314 RSC.
-
(a) X.-F. Huang, Y.-F. Zhang, Z.-H. Qi, N.-K. Li, Z.-C. Geng, K. Li and X.-W. Wang, Org. Biomol. Chem., 2014, 12, 4372 RSC;
(b) V. P. R. Gajulapalli, P. Vinayagam and V. Kesavan, RSC Adv., 2015, 5, 7370 RSC.
- T.-Z. Li, J. Xie, Y. Jiang, F. Sha and X.-Y. Wu, Adv. Synth. Catal., 2015, 357, 3507 CrossRef CAS.
- J.-S. Yu, F.-M. Liao, W.-M. Gao, K. Liao, R.-L. Zuo and J. Zhou, Angew. Chem., Int. Ed., 2015, 54, 7381 CrossRef CAS PubMed.
- K. Higashiyama and H. Otomasu, Chem. Pharm. Bull., 1980, 28, 1540 CrossRef CAS.
- F. Zhong, C. Jiang, W. Yao, L.-W. Xu and Y. Lu, Tetrahedron Lett., 2013, 54, 4333 CrossRef CAS.
- P. T. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686 CrossRef CAS PubMed and references therein.
- ESI.†.
- H. Y. Bae, J. H. Sim, J.-W. Lee, B. List and C. E. Song, Angew. Chem., Int. Ed., 2013, 52, 12143 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization data for new compounds, X-ray crystal structure and the CIF file of 4g and 5g. CCDC 1481433 (5g) and 1481435 (4g). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra16975a |
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 weight ratio of silica gel to crude product. β-keto acids 3 and Malonic acid half thioesters 6 were prepared according to the literature procedure.13,17
1 weight ratio of silica gel to crude product. β-keto acids 3 and Malonic acid half thioesters 6 were prepared according to the literature procedure.13,17
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the pure products 4.
1) to afford the pure products 4.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 2
1 to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford the pure products 5.
1) to afford the pure products 5.








