Direct determination of total mercury in urine samples using flow injection catalytic cold vapor atomic absorption spectrometry (FI-CCV-AAS)
Abstract
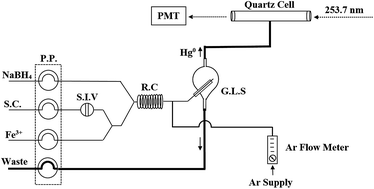
A rapid flow injection catalytic cold vapor atomic absorption spectrometric (FI-CCV-AAS) method is described for determination of total mercury in urine samples. In this work, instead of preliminary oxidation or digestion steps, efficiency of vaporization of organic mercury was increased using Fe3+ as the catalyst directly in line in the flow injection protocol, therefore sample pretreatment was eliminated. Several physical and chemical variables in the flow injection system were studied and optimized in order to generate identical analytical signals for both inorganic and organic mercury. Due to the similarity of the resulting sensitivity, Hg2+ was used successfully as a primary standard for calibration. Limit of detection, coefficient of regression and linear dynamic range were obtained as 0.14 μg L−1, 0.9997 and 0.50–35.0 μg L−1, respectively. The use of flow injection system enabled fast analysis with a sample throughput of 108 h−1. The application of the method to the quantification of total mercury in the two levels of urine reference material (SRM 3668) gave 107.7% and 97.3% recoveries for lower and elevated levels, respectively. The method successfully recovered spiked Hg2+ and methylmercury in 101 urine samples in the range of 93.4–104.5%.

 Please wait while we load your content...
Please wait while we load your content...