Highly stable MIL-101(Cr) doped water permeable thin film nanocomposite membranes for water treatment†
Abstract
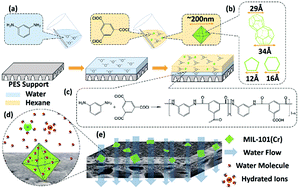
Highly stable water permeable thin film MIL-101(Cr) nanocomposite membranes for water treatment were created via in situ interfacial polymerization on a polyether sulfone support. Lab-made MIL-101(Cr) nanoparticles (∼200 nm) were used to introduce direct water channels to a dense polyamide layer, which increased water permeance. On changing the amount and the method of MIL-101(Cr) nanoparticle addition, the surface topography and internal structure of the membranes were changed, leading to different separating properties of the membranes. Measured at 10 bar with 2000 ppm Na2SO4 solution, nanosized MIL-101(Cr) increased water permeance to 3.91 L m−2 h−1 bar−1 at 0.2 w/v%, 158% higher than undoped membranes; meanwhile, high Na2SO4 rejection was maintained. This study experimentally verified the potential use of MIL-101(Cr) in advanced thin film nanocomposite membranes, which can be used in diversified water purification fields, including desalination and the removal of organics.

 Please wait while we load your content...
Please wait while we load your content...