Spiro-condensation of 5-methoxycarbonyl-1H-pyrrole-2,3-diones with cyclic enoles to form spiro substituted furo[3,2-c]-coumarins and quinolines†
Alexey Yu. Dubovtseva,
Pavel S. Silaicheva,
Mikhail A. Nazarova,
Maksim V. Dmitrieva,
Andrey N. Maslivets*a and
Michael Rubin *bc
*bc
aDepartment of Chemistry, Perm State University, ul. Bukireva 15, Perm 614990, Russia. E-mail: koh2@psu.ru
bDepartment of Chemistry, North Caucasus Federal University, 1a Pushkin St., Stavropol 355009, Russian Federation
cDepartment of Chemistry, University of Kansas, 1251 Wescoe Hall Dr., Lawrence, KS 66045-7582, USA. E-mail: mrubin@ku.edu; Fax: +1 785 864 5396; Tel: +1 785 864 5071
First published on 2nd September 2016
Abstract
Highly efficient spiro-condensation enabling cyclic enoles to act as 1,3-bis-nucleophiles in reaction with pyrrole-2,3-diones acting as 1,2-bis-electrophiles was developed. The corresponding furo[3,2-c]coumarins and furo[3,2-c]quinolines containing a spiro pyrrole fragment were obtained in high yields.
Introduction
Natural products containing furo[3,2-c]coumarin1 and furo[3,2-c]quinolone2 fragments have been the subject of great interest due to their important biological properties. For example, phyto-alkaloids hypocrolide A, glaumacidine, coumestrol (coumestan), and their synthetic analogs demonstrated promising antimitotic, antiprolifirative and cytotoxic activities (Scheme 1). Not surprisingly, development of new synthetic routes to these scaffolds is of great interest for modern synthetic and medicinal chemistry. Herein we wish to disclose the results of our synthetic studies towards spiro analogs of furo[3,2-c]coumarins and furo[3,2-c]quinolones. The featured synthetic strategy is based on highly selective electrophilic reactions of 1H-pyrrole-2,3-diones.Results and discussion
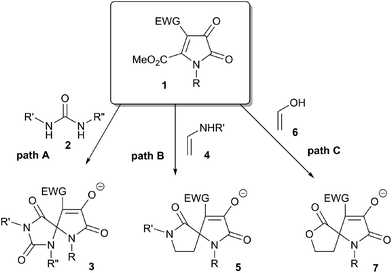
Due to their high reactivity and pronounced electrophilic properties 1H-pyrrole-2,3-diones are often employed as useful building blocks allowing for efficient incorporation of a nitrogen containing five-membered heterocyclic unit in the structure of a target molecule. Such approach has found application in total synthesis of natural alkaloids.3 The installation of additional electron-withdrawing groups (such as acyl, alkoxycarbonyl or 1,2-dicarbonyl moieties) at C-4 and C-5 of the pyrrole ring may further increase synthetic versatility of these compounds. Diverse reactions of such 4,5-disubstituted 1H-pyrrole-2,3-diones can be used for expeditious assembly of various fused and bridged, polyheterocyclic scaffolds, often hardly available or unavailable by other methods.4 Furthermore, the installation of an ester function at C-5 provides an additional electrophilic moiety in multistep cascade reactions with bis-nucleophilic reagents, offering easy access towards functionalized spiro-pyrroles (Scheme 2). Thus, we previously demonstrated the reaction of 5-methoxycarbonyl-1H-pyrrole-2,3-diones 1 with 1,3-N,N-bis-nuclephiles, such as urea derivatives 2 to yield derivatives of 1,3,6-triazaspiro[4,4]nonane 3 (Scheme 2, path A).5 Similarly, utilization of enamines 4 as 1,3-C,N-bisnucleophiles afforded products with 1,7-diazaspiro[4,4]nonane scaffold 5 (Scheme 2, path B).6,7 A detailed investigation of this transformation revealed that both acyclic6 and cyclic7 enamines can be readily employed as bis-nucleophiles, providing facile access to annulated functionalized spiranes 5 with increased molecular complexity. At the same time, to the best of our knowledge, related spiro-condensations of monocyclic 1H-pyrrole-2,3-diones 1 with enoles 6 (Scheme 2, path C) is still unknown. We envisioned that such a process, if successful, might serve for efficient preparation of 7-oxa-1-azaspiro[4,4]nonanes 7, including spiro-derivatives of furo[3,2-c]coumarins and furo[3,2-c]quinolones.To this end we envisioned that stable enol forms such as 4-hydroxycoumarine (6a, X = O) and 4-hydroxyquinolin-2(1H)-ones (6b, X = NMe; 6b, X = NPh) could serve as suitable precursors for the designed transformation. Indeed, enolate 8 generated in the presence of catalytic base is expected to perform a conjugate addition across the highly electrophilic vinylogous amide moiety in pyrroledione 1. The resulting enolate 9 is anticipated to undergo a proton transfer to produce less basic enolate 10, which is well suited for an intramolecular 5-exo-trig cyclization affecting the ester function at C-5 and providing target structure 11 (Scheme 3).
To evaluate this idea we carried out the reaction of methyl 3-benzoyl-4,5-dioxo-1-phenyl-4,5-dihydro-1H-pyrrole-2-carboxylate (1a, Ar1 = Ph, Ar2 = Ph) with 4-hydroxy-2H-chromen-2-one (6a). It was observed that an equimolar mixture of these starting materials when refluxed in toluene in the presence of catalytic amounts of triethylamine (10 mol%) engaged in a quick reaction. The distinct purple color of pyrroledione faded away and the colorless product 11aa crystallized directly from the reaction mixture after cooling it down to −10 °C. This furo-coumarine was obtained as the sole product in good yield (68%, Table 1, entry 1). Reactions of other 4-aroyl-5-methoxycarbonyl pyrrolediones with 6a also proceeded uneventfully, affording the corresponding furo[3,2-c]coumarins very efficiently (Table 1, entries 2–8). Reactions involving enolates generated from 4-hydroxy-2-quinolones 6b, c were carried out under the same conditions and also afforded the corresponding spiro furo[3,2-c]quinolones as sole products, albeit in somewhat lower yields (Table 1, entries 9–13). Formation of these structures was unambiguously confirmed by X-ray crystallography of compounds 11ba (Fig. 1) and 11db (Fig. 2).
| # | 1 | 6 | X | Ar1 | Ar2 | 11 | Yielda, % |
|---|---|---|---|---|---|---|---|
| a Isolated yields of purified compounds 11. | |||||||
| 1 | 1a | 6a | O | Ph | Ph | 11aa | 68 |
| 2 | 1b | 6a | O | 4-MeOC6H4 | Ph | 11ba | 70 |
| 3 | 1c | 6a | O | Ph | 4-MeOC6H4 | 11ca | 84 |
| 4 | 1d | 6a | O | Ph | 4-MeC6H4 | 11da | 80 |
| 5 | 1e | 6a | O | 4-MeC6H4 | Ph | 11ea | 76 |
| 6 | 1f | 6a | O | 4-MeC6H4 | 4-MeC6H4 | 11fa | 78 |
| 7 | 1g | 6a | O | Ph | 4-ClC6H4 | 11ga | 67 |
| 8 | 1h | 6a | O | Ph | 4-BrC6H4 | 11ha | 76 |
| 9 | 1c | 6b | NMe | Ph | 4-MeOC6H4 | 11cb | 68 |
| 10 | 1d | 6b | NMe | Ph | 4-MeC6H4 | 11db | 69 |
| 11 | 1a | 6c | NPh | Ph | Ph | 11ac | 56 |
| 12 | 1g | 6c | NPh | Ph | 4-ClC6H4 | 11gc | 59 |
| 13 | 1i | 6c | NPh | 4-BrC6H4 | 4-MeC6H4 | 11ic | 67 |
 | ||
| Fig. 1 ORTEP drawing of compound 11ba: showing 50% probability amplitude displacement ellipsoids (CCDC # 1486439†). | ||
 | ||
| Fig. 2 ORTEP drawing of compound 11db: showing 50% probability amplitude displacement ellipsoids and a molecule of disordered crystallized solvent (acetonitrile) (CCDC # 1486440†). | ||
We wondered, if the featured spiro-condensation would proceed selectively in more complex substrates, possibly allowing for alternative reaction pathways. For example, reaction of pyrrolediones 12 bearing a cinnamoyl substituent at C-4, with bis-nucleophilic enolates should proceed through intermediate 14, which could in principle undergo a second-fold enolexo-6-endo-trig Michael addition reaction to furnish different spiro scaffold 15 (Scheme 4). In our recent report we disclosed that in reactions of 4-cinnamoyl-pyrrole-2,3-diones 12 with five-membered cyclic enolates this process was prevalent.8 Interestingly, in the presence of six-membered enolates, generated from heterocyclic precursors 6a–c the reaction did not take this alternative route at all, proceeding instead via a “normal” 5-exo-trig lactonization pathway to afford the corresponding furo[3,2-c]coumarins and furo[3,2-c]quinolones 13 as sole products (Scheme 4, Table 2).
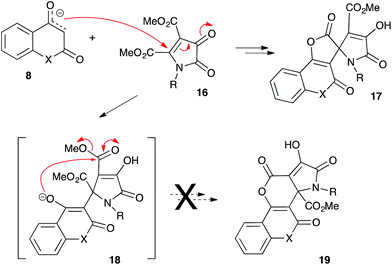
Next, we investigated the reactivity of 4,5-dimethoxycarbonyl-1H-pyrrole-2,3-diones 16. After initial Michael addition of cyclic enolate 8, these substrates should provide intermediate 18, which can undergo the subsequent intramolecular nucleophilic attack involving one of the two available ester groups (Scheme 5). We were pleased to discover that these reactions also proceeded chemoselectively according to 5-exo-trig pathway, providing γ-lactones 17 as sole products in high yields (Table 3, entries 1–6). The alternative 6-exo-trig pathway was not realized at all, and the corresponding δ-lactones 18 were not detected in the reaction mixtures (Scheme 5).
Conclusions
We have developed a novel cascade transformation combining an intermolecular conjugate addition of stabilized cyclic enolate (4-hydroxycoumarin or 4-hydroxy-2-quinolone) across a highly electrophilic vinylogous amide moiety of pyrrole-2,3-dione, and subsequent lactonization involving intramolecular nucleophilic attack of O-enolate at the ester substituent at C-5. The corresponding spiro furo[3,2-c]coumarins and furo[3,2-c]quinolones were formed as sole products in good yields. Remarkably, the introduction of competitive electrophilic substituents at C-4, such as cinnamoyl or methoxycarbonyl groups, did not divert the reaction from the described mechanistic route.Experimental part
1H and 13C NMR spectra were recorded on a Bruker Avance-III spectrometer (400 or 100 MHz, respectively) equipped with BBO probe in CDCl3 or DMSO-d6 using TMS as internal standard. IR spectra were recorded with a Perkin–Elmer Spectrum Two spectrometer from mulls in mineral oil. Melting points were measured with Stuart smp30 apparatus. X-ray crystallography was performed on Xcalibur Ruby diffractometer. The mass spectra were recorded on an Waters UPLC-MS instrument equipped with an ESI MS Xevo TQD detector. Elemental analyses were carried out on Vario MICRO Cube analyzer. Starting 5-methoxycarbonyl 1H-pyrrole-2,3-diones 1a–i, 12a–d, and 16a–c were obtained as described in literature sources,9 N-methyl- and N-phenyl-4-hydroxy-2-quinolones 6b, c were also prepared according to literature procedures.10 Anhydrous toluene was obtained by heating at reflux with molten sodium followed by distillation in under an atmosphere of dry nitrogen. Other reagents and solvents were purchased from commercial vendors and were used as received.3′-Benzoyl-4′-hydroxy-1′-(4-methoxyphenyl)-2H,4H-spiro[furo[3,2-c]chromene-3,2′-pyrrole]-2,4,5′(1′H)-trione (11ca, typical procedure)
A solution of 4-hydroxycoumarin 6a (162 mg, 1.00 mmol), methyl 3-benzoyl-1-(4-methoxyphenyl)-4,5-dioxo-4,5-dihydro-1H-pyrrole-2-carboxylate 1c (365 mg, 1.00 mmol), and Et3N (10 mg, 0.10 mmol) in anhydrous toluene (5 mL) was stirred at reflux for 1.5 h until purple color of pyrroledione faded away, and the solid precipitate formed. Then the reaction mixture was cooled to −10 °C, and the resulted precipitate was filtered off, washed with hexane and recrystallized from toluene/chloroform (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to afford 11ca (416 mg, 84%) as a colorless crystals, mp 240–242 °C (dec.). 1H NMR (400 MHz, CDCl3) δ 7.88–7.85 (m, 2H, Ar), 7.76–7.71 (m, 1H, Ar), 7.67–7.53 (m, 2H, Ar), 7.48–7.31 (m, 4H, Ar), 7.19–7.13 (m, 2H, Ar), 6.87–6.83 (m, 2H, Ar), 3.74 (s, 3H, OMe); 13C NMR (100 MHz, CDCl3) δ 188.9, 169.7, 166.2, 165.4, 160.5, 156.1, 155.9, 150.4, 136.5, 134.9, 133.9, 129.6, 129.0, 128.6, 125.8, 125.1, 123.9, 117.7, 116.6, 115.5, 110.3, 100.9, 70.0, 55.6; IR (NaCl, cm−1): 3408, 1848, 1736, 1707, 1676, 1652; MS: found 496.17; calcd for C28H18NO8 (M + H)+ 496.10; EA: found C 67.84, H 3.45, N 2.86; calcd for C28H17NO8 (495.44): C 67.88, H 3.46, N 2.83.
1) to afford 11ca (416 mg, 84%) as a colorless crystals, mp 240–242 °C (dec.). 1H NMR (400 MHz, CDCl3) δ 7.88–7.85 (m, 2H, Ar), 7.76–7.71 (m, 1H, Ar), 7.67–7.53 (m, 2H, Ar), 7.48–7.31 (m, 4H, Ar), 7.19–7.13 (m, 2H, Ar), 6.87–6.83 (m, 2H, Ar), 3.74 (s, 3H, OMe); 13C NMR (100 MHz, CDCl3) δ 188.9, 169.7, 166.2, 165.4, 160.5, 156.1, 155.9, 150.4, 136.5, 134.9, 133.9, 129.6, 129.0, 128.6, 125.8, 125.1, 123.9, 117.7, 116.6, 115.5, 110.3, 100.9, 70.0, 55.6; IR (NaCl, cm−1): 3408, 1848, 1736, 1707, 1676, 1652; MS: found 496.17; calcd for C28H18NO8 (M + H)+ 496.10; EA: found C 67.84, H 3.45, N 2.86; calcd for C28H17NO8 (495.44): C 67.88, H 3.46, N 2.83.
3′-Benzoyl-4′-hydroxy-1′-phenyl-2H,4H-spiro[furo[3,2-c]chromene-3,2′-pyrrole]-2,4,5′(1′H)-trione (11aa)
Yield 316 mg (68%), mp 237–239 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 7.89–7.85 (m, 2H, Ar), 7.73 (ddd, J = 7.9, 1.6, 0.5 Hz, 1H, Ar), 7.65–7.53 (m, 2H, Ar), 7.46–7.40 (m, 2H, Ar), 7.38–7.30 (m, 5H, Ar), 7.27–7.24 (m, 2H, Ar); 13C NMR (100 MHz, CDCl3) δ 188.9, 169.6, 166.0, 165.4, 156.0, 155.9, 150.4, 136.5, 134.9, 133.9, 133.5, 130.2, 129.8, 129.6, 128.6, 127.5, 125.1, 123.9, 117.7, 116.7, 110.3, 100.8, 69.7 ppm. IR (NaCl, cm−1): 3413, 1851, 1744, 1711, 1674, 1648; MS: found 466.15; calcd for C27H16NO7 (M + H)+ 466.09; EA: found C 69.64, H 3.23, N 3.04; calcd for C27H15NO7 (465.42): C 69.68, H 3.25, N 3.01.4′-Hydroxy-3′-(4-methoxybenzoyl)-1′-phenyl-2H,4H-spiro[furo[3,2-c]chromene-3,2′-pyrrole]-2,4,5′(1′H)-trione (11ba)
Yield 347 mg (70%), mp 251–252 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 7.92–7.87 (m, 2H, Ar), 7.78–7.71 (m, 1H, Ar), 7.77 (ddd, J = 8.4, 7.5, 1.6 Hz, 1H, Ar), 7.41–7.23 (m, 7H, Ar), 6.97–6.92 (m, 2H, Ar), 3.87 (s, 3H, OMe); 13C NMR (100 MHz, CDCl3) δ 186.9, 169.7, 166.0, 165.4, 164.6, 156.0, 155.9, 148.9, 134.9, 133.7, 132.3, 130.2, 129.7, 129.2, 127.5, 125.1, 123.9, 177.7, 117.1, 114.1, 110.3, 100.9, 65.9, 55.7; IR (NaCl, cm−1): 3428, 1848, 1737, 1717, 1671, 1651; MS: found 496.19; calcd for C28H18NO8 (M + H)+ 496.10; EA: found C 67.83, H 3.46, N 2.84; calcd for C28H17NO8 (495.44): C 67.88, H 3.46, N 2.83.3′-Benzoyl-4′-hydroxy-1′-(4-tolyl)-2H,4H-spiro[furo[3,2-c]chromene-3,2′-pyrrole]-2,4,5′(1′H)-trione (11da)
Yield 383 mg (80%), mp 203–205 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 7.92–7.87 (m, 2H, Ar), 7.78–7.71 (m, 1H, Ar), 7.68–7.53 (m, 2H, Ar), 7.48–7.40 (m, 2H, Ar), 7.37–7.30 (m, 2H, Ar), 7.19–7.06 (m, 4H, Ar), 2.21 (s, 3H, Me); 13C NMR (100 MHz, CDCl3) δ 188.9, 169.7, 166.1, 165.4, 156.0, 155.9, 150.5, 140.1, 136.5, 134.9, 133.9, 130.8, 130.8, 129.6, 128.6, 127.3, 125.0, 123.9, 117.7, 116.6, 110.3, 100.9, 69.8, 21.3; IR (NaCl, cm−1): 3441, 1851, 1724, 1708, 1674, 1646; MS: found 480.19; calcd for C28H18NO7 (M + H)+ 480.11; EA: found C 70.12, H 3.56, N 2.94; calcd for C28H17NO7 (479.44): C 70.15, H 3.57, N 2.92.4′-Hydroxy-3′-(4-methylbenzoyl)-1′-phenyl-2H,4H-spiro[furo[3,2-c]chromene-3,2′-pyrrole]-2,4,5′(1′H)-trione (11ea)
Yield 364 mg (76%), mp 229–231 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 7.81–7.71 (m, 3H, Ar), 7.63 (ddd, J = 8.4, 7.5, 1.6 Hz, 1H, Ar), 7.41–7.30 (m, 5H, Ar), 7.28–7.21 (m, 4H, Ar), 2.40 (s, 3H, Me); 13C NMR (100 MHz, CDCl3) δ 188.4, 169.7, 166.1, 165.4, 156.0, 155.9, 149.9, 145.2, 134.9, 133.8, 133.5, 130.2, 129.8, 129.8, 129.4, 127.5, 124.9, 123.9, 117.7, 117.0, 110.2, 100.7, 69.7, 22.0; IR (NaCl, cm−1): 3420, 1854, 1713, 1673, 1648; MS: found 480.18; calcd for C28H18NO7 (M + H)+ 480.11; EA: found C 70.11, H 3.55, N 2.92; calcd for C28H17NO7 (479.44): C 70.15, H 3.57, N 2.92.4′-Hydroxy-3′-(4-methylbenzoyl)-1′-(4-tolyl)-2H,4H-spiro[furo[3,2-c]chromene-3,2′-pyrrole]-2,4,5′(1′H)-trione (11fa)
Yield 385 mg (78%), mp 259–260 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 7.80–7.71 (m, 3H, Ar), 7.63 (ddd, J = 8.3, 7.5, 1.6 Hz, 1H, Ar), 7.38–7.29 (m, 2H, Ar), 7.27–7.22 (m, 3H, Ar), 7.17–7.11 (m, 3H, Ar), 2.40 (s, 3H, Me), 2.29 (s, 3H, Me); 13C NMR (100 MHz, CDCl3) δ 188.3, 169.8, 166.0, 165.3, 155.9, 149.8, 145.1, 140.0, 134.8, 133.9, 130.8, 129.8, 129.4, 127.3, 125.0, 123.9, 117.7, 116.7, 110.4, 101.0, 69.8, 22.0, 21.3; IR (NaCl, cm−1): 3438, 1848, 1723, 1673, 1646; MS: found 494.23; calcd for C29H20NO7 (M + H)+ 494.12; EA: found C 70.54, H 3.86, N 2.85; calcd for C29H19NO7 (493.47): C 70.59, H 3.88, N 2.84.3′-Benzoyl-1′-(4-chlorophenyl)-4′-hydroxy-2H,4H-spiro[furo[3,2-c]chromene-3,2′-pyrrole]-2,4,5′(1′H)-trione (11ga)
Yield 335 mg (67%), mp 233–235 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 7.88–7.84 (m, 2H, Ar), 7.77 (dd, J = 7.9, 1.6 Hz, 1H, Ar), 7.70–7.62 (m, 1H, Ar), 7.58 (t, J = 7.4 Hz, 1H, Ar), 7.45 (t, J = 7.7 Hz, 2H, Ar), 7.40–7.30 (m, 4H, Ar), 7.20 (dd, J = 9.1, 2.4 Hz, 2H, Ar); 13C NMR (100 MHz, CDCl3) δ 188.8, 169.6, 165.9, 165.6, 156.0 (2C), 150.0, 136.4, 136.0, 135.2, 134.1, 132.0, 130.5, 129.6, 128.9, 128.7, 125.2, 124.0, 117.8, 116.9, 110.2, 100.5, 69.6; IR (NaCl, cm−1): 3464, 1849, 1737, 1709, 1680, 1656; MS: found 500.17; calcd for C27H15ClNO7 (M + H)+ 500.05; EA: found C 64.83, H 2.80, Cl 7.12, N 2.81; calcd for C27H14ClNO7 (499.86): C 64.88, H 2.82, Cl 7.09, N 2.80.3′-Benzoyl-1′-(4-bromophenyl)-4′-hydroxy-2H,4H-spiro[furo[3,2-c]chromene-3,2′-pyrrole]-2,4,5′(1′H)-trione (11ha)
Yield 413 mg (76%), mp 235–236 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 7.88–7.83 (m, 2H, Ar), 7.77 (dd, J = 7.9, 1.6 Hz, 1H, Ar), 7.66 (ddd, J = 8.9, 7.5, 1.6 Hz, 1H, Ar), 7.58 (ddd, J = 8.7, 2.5, 1.2 Hz, 1H, Ar), 7.52–7.41 (m, 4H, Ar), 7.39–7.33 (m, 2H, Ar), 7.16–7.11 (m, 2H, Ar); 13C NMR (100 MHz, CDCl3) δ 188.8, 169.6, 165.8, 165.6, 156.0, 156.0, 149.9, 136.4, 135.2, 134.1, 133.5, 132.6, 129.6, 129.1, 128.7, 125.2, 124.1, 124.0, 117.8, 116.9, 110.2, 100.5, 69.6; IR (NaCl, cm−1): 3473, 1848, 1738, 1709, 1678, 1654; MS: found 544.18; calcd for C27H15BrNO7 (M + H)+ 544.00; EA: found C 59.54, H 2.61, Br 14.73, N 2.57; calcd for C27H14BrNO7 (544.31): C 59.58, H 2.59, Br 14.68, N 2.57.3′-Benzoyl-4′-hydroxy-1′-(4-methoxyphenyl)-5-methyl-2H-spiro[furo[3,2-c]quinoline-3,2′-pyrrole]-2,4,5′(1′H,5H)-trione (11cb)
Yield 345 mg (68%), mp 243–244 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 7.92–7.84 (m, 3H, Ar), 7.66 (ddd, J = 8.8, 7.2, 1.6 Hz, 1H, Ar), 7.56–7.47 (m, 1H, Ar), 7.42–7.28 (m, 4H, Ar), 7.22–7.14 (m, 2H, Ar), 6.85–6.80 (m, 2H, Ar), 3.74 (s, 3H, Me), 3.63 (s, 3H, OMe); 13C NMR (100 MHz, CDCl3) δ 188.9, 171.2, 166.0, 161.0, 160.2, 157.7, 150.2, 141.9, 136.9, 133.5, 133.5, 129.7, 129.1, 128.5, 126.4, 124.2, 122.8, 116.6, 115.2, 115.1, 110.5, 105.6, 70.9, 55.5, 29.4; IR (NaCl, cm−1): 3429, 1838, 1738, 1727, 1659, 1630; MS: found 509.21; calcd for C29H21N2O7 (M + H)+ 509.13; EA: found C 68.44, H 3.93, N 5.56; calcd for C29H20N2O7 (508.49): C 68.50, H 3.96, N 5.51.3′-Benzoyl-4′-hydroxy-5-methyl-1′-(4-tolyl)-2H-spiro[furo[3,2-c]quinoline-3,2′-pyrrole]-2,4,5′(1′H,5H)-trione (11db)
Yield 339 mg (69%), mp 257–259 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 7.92–7.82 (m, 3H, Ar), 7.68 (ddd, J = 8.8, 7.2, 1.6 Hz, 1H, Ar), 7.54–7.49 (m, 1H, Ar), 7.40–7.26 (m, 4H, Ar), 7.18–7.09 (m, 4H, Ar), 3.62 (s, 3H, Me), 2.28 (s, 3H, C6H4Me); 13C NMR (100 MHz, DMSO-d6) δ 188.5, 171.7, 165.6, 159.2, 156.6, 153.8, 141.0, 138.7, 136.6, 133.6, 133.1, 131.4, 130.2, 128.9, 128.2, 126.6, 122.8 (2C), 116.0, 115.4, 108.9, 105.4, 69.4, 28.9, 20.5; IR (NaCl, cm−1): 3449, 1837, 1738, 1659, 1634; MS: found 493.21; calcd for C29H21N2O6 (M + H)+ 493.14; EA: found C 70.64, H 4.08, N 5.73; calcd for C29H20N2O6 (492.49): C 70.73, H 4.09, N 5.69.3′-Benzoyl-4′-hydroxy-1′,5-diphenyl-2H-spiro[furo[3,2-c]quinoline-3,2′-pyrrole]-2,4,5′(1′H,5H)-trione (11ac)
Yield 303 mg (56%), mp 261–263 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 7.89–7.81 (m, 3H, Ar), 7.62–7.46 (m, 4H, Ar), 7.45–7.28 (m, 8H, Ar), 7.23 (d, J = 7.2 Hz, 1H, Ar), 7.15–7.12 (m, 1H, Ar), 7.03 (d, J = 7.7 Hz, 1H, Ar), 6.67 (d, J = 8.6 Hz, 1H, Ar); 13C NMR (100 MHz, CDCl3) δ 189.1, 171.1, 166.0, 161.6, 157.6, 150.3, 143.0, 136.9, 136.6, 134.2, 133.6, 133.0, 130.4, 129.9, 129.6, 129.5, 129.4, 129.2, 128.8, 128.6, 127.6, 123.7, 123.0, 116.9, 110.2, 105.8, 70.6; IR (NaCl, cm−1): 3443, 1835, 1732, 1718, 1637, 1626; MS: found 541.21; calcd for C33H21N2O6 (M + H)+ 541.14; EA: found C 73.29, H 3.71, N 5.20; calcd for C33H20N2O6 (540.53): C 73.33, H 3.73, N 5.18.3′-Benzoyl-1′-(4-chlorophenyl)-4′-hydroxy-5-phenyl-2H-spiro[furo[3,2-c]quinoline-3,2′-pyrrole]-2,4,5′(1′H,5H)-trione (11gc)
Yield 339 mg (59%), mp 229–231 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 7.90–7.84 (m, 3H, Ar), 7.62–7.48 (m, 4H, Ar), 7.42 (ddd, J = 7.3, 4.7, 1.0 Hz, 3H, Ar), 7.37–7.22 (m, 5H, Ar), 7.16–7.10 (m, 1H, Ar), 7.07–7.01 (m, 1H, Ar), 6.69 (d, J = 8.5 Hz, 1H, Ar); 13C NMR (100 MHz, CDCl3) δ 189.0, 170.9, 165.9, 161.7, 157.6, 149.8, 143.0, 136.8, 136.5, 135.6, 133.8, 133.2, 132.7, 130.4, 130.2, 129.6, 129.5, 129.2, 129.0, 128.8, 128.6, 123.8, 123.2, 117.0, 116.8, 110.2, 105.3, 70.6; IR (NaCl, cm−1): 3400, 1841, 1734, 1651, 1622; MS: found 575.23; calcd for C33H20ClN2O6 (M + H)+ 575.10; EA: found C 68.89, H 3.46, Cl 6.11, N 4.80; calcd for C33H19ClN2O6 (574.97): C 68.94, H 3.33, Cl 6.17, N 4.87.3′-(4-Bromobenzoyl)-4′-hydroxy-5-phenyl-1′-(4-tolyl)-2H-spiro[furo[3,2-c]quinoline-3,2′-pyrrole]-2,4,5′(1′H,5H)-trione (11ic)
Yield 362 mg (67%), mp 272–274 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 7.85 (d, J = 8.0 Hz, 1H, Ar), 7.76 (d, J = 8.3 Hz, 2H, Ar), 7.60–7.47 (m, 3H, Ar), 7.42–7.36 (m, 3H, Ar), 7.28–7.11 (m, 6H, Ar), 7.05 (d, J = 7.0 Hz, 1H, Ar), 6.69 (d, J = 8.7 Hz, 1H, Ar), 2.33 (s, 3H, Me); 13C NMR (100 MHz, CDCl3) δ 188.2, 177.1, 165.7, 161.7, 157.6, 151.1, 142.9, 139.8, 136.5, 135.8, 133.0, 131.8, 131.3, 130.7, 130.4, 130.4, 129.9, 129.5, 129.3, 128.8, 128.5, 127.8, 123.7, 123.1, 120.1, 117.1, 116.3, 110.3, 105.8, 70.5, 21.4; IR (NaCl, cm−1): 3449, 1836, 1735, 1660, 1616; MS: found 633.15; calcd for C34H21BrN2O6 (M + H)+ 633.07; EA: found C 64.36, H 3.39, Br 12.43, N 4.37; calcd for C34H21BrN2O6 (633.45): C 64.47, H 3.34, Br 12.61, N 4.42.3′-Cinnamoyl-4′-hydroxy-1′-(4-tolyl)-2H,4H-spiro[furo[3,2-c]chromene-3,2′-pyrrole]-2,4,5′(1′H)-trione (13aa)
Yield 339 mg (67%), mp 236–237 °C (dec.), colorless crystals. 1H NMR (400 MHz, DMSO-d6) δ 11.25 (br. s, 1H, OH), 7.88–7.83 (m, 2H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.75–7.57 (m, 5H, Ar + CH
CH), 7.75–7.57 (m, 5H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.55–7.39 (m, 4H, Ar + CH
CH), 7.55–7.39 (m, 4H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.25 (d, J = 7.3 Hz, 2H, Ar + CH
CH), 7.25 (d, J = 7.3 Hz, 2H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.02 (d, J = 7.4 Hz, 2H, Ar + CH
CH), 7.02 (d, J = 7.4 Hz, 2H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 2.24 (s, 3H, Me); 13C NMR (100 MHz, DMSO-d6) δ 182.0, 170.9, 165.8, 163.5, 157.6, 155.2, 154.8, 143.1, 139.1, 135.4, 133.4, 131.2, 130.8, 130.6, 129.1, 128.5, 126.6, 125.6, 123.3, 117.5, 115.6, 109.3, 101.8, 67.9, 20.6; IR (NaCl, cm−1): 3420, 1843, 1716, 1670, 1642; MS: found 506.25; calcd for C30H20NO7 (M + H)+ 506.12; EA: found C 71.36, H 3.73, N 2.79; calcd for C30H19NO7 (505.48): C 71.28, H 3.79, N 2.77.
CH), 2.24 (s, 3H, Me); 13C NMR (100 MHz, DMSO-d6) δ 182.0, 170.9, 165.8, 163.5, 157.6, 155.2, 154.8, 143.1, 139.1, 135.4, 133.4, 131.2, 130.8, 130.6, 129.1, 128.5, 126.6, 125.6, 123.3, 117.5, 115.6, 109.3, 101.8, 67.9, 20.6; IR (NaCl, cm−1): 3420, 1843, 1716, 1670, 1642; MS: found 506.25; calcd for C30H20NO7 (M + H)+ 506.12; EA: found C 71.36, H 3.73, N 2.79; calcd for C30H19NO7 (505.48): C 71.28, H 3.79, N 2.77.
3′-Cinnamoyl-4′-hydroxy-5-methyl-1′-(4-tolyl)-2H-spiro[furo[3,2-c]quinoline-3,2′-pyrrole]-2,4,5′(1′H,5H)-trione (13ab)
Yield 389 mg (75%), mp 276–277 °C (dec.), colorless crystals. 1H NMR (400 MHz, DMSO-d6) δ 7.83–7.75 (m, 2H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.67–7.60 (m, 5H, Ar + CH
CH), 7.67–7.60 (m, 5H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.49–7.34 (m, 4H, Ar + CH
CH), 7.49–7.34 (m, 4H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.18 (d, J = 8.1 Hz, 2H, Ar + CH
CH), 7.18 (d, J = 8.1 Hz, 2H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.04–6.99 (m, 2H, Ar + CH
CH), 7.04–6.99 (m, 2H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.59 (s, 3H, Me), 2.21 (s, 3H, C6H4Me); 13C NMR (100 MHz, DMSO-d6) δ 181.9, 171.8, 165.6, 158.9, 156.5, 156.5, 142.9, 140.9, 138.6, 134.2, 133.4, 131.5, 130.7, 130.1, 129.0, 128.3, 126.5, 123.4, 122.8, 122.7, 116.4, 115.9, 109.0, 106.1, 68.7, 28.8, 20.4; IR (NaCl, cm−1): 3437, 1841, 1728, 1667, 1639; MS: found 519.35; calcd for C31H23N2O6 (M + H)+ 519.16; EA: found C 71.76, H 4.21, N 5.43; calcd for C31H22N2O6 (518.53): C 71.81, H 4.28, N 5.40.
CH), 3.59 (s, 3H, Me), 2.21 (s, 3H, C6H4Me); 13C NMR (100 MHz, DMSO-d6) δ 181.9, 171.8, 165.6, 158.9, 156.5, 156.5, 142.9, 140.9, 138.6, 134.2, 133.4, 131.5, 130.7, 130.1, 129.0, 128.3, 126.5, 123.4, 122.8, 122.7, 116.4, 115.9, 109.0, 106.1, 68.7, 28.8, 20.4; IR (NaCl, cm−1): 3437, 1841, 1728, 1667, 1639; MS: found 519.35; calcd for C31H23N2O6 (M + H)+ 519.16; EA: found C 71.76, H 4.21, N 5.43; calcd for C31H22N2O6 (518.53): C 71.81, H 4.28, N 5.40.
3′-Cinnamoyl-4′-hydroxy-1′-(4-methoxyphenyl)-5-methyl-2H-spiro[furo[3,2-c]quinoline-3,2′-pyrrole]-2,4,5′(1′H,5H)-trione (13bb)
Yield 417 mg (78%), mp 297–298 °C (dec.), colorless crystals. 1H NMR (400 MHz, DMSO-d6) δ 7.83–7.75 (m, 2H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.74–7.51 (m, 5H, Ar + CH
CH), 7.74–7.51 (m, 5H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.49–7.36 (m, 4H, Ar + CH
CH), 7.49–7.36 (m, 4H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.07–7.00 (m, 2H, Ar + CH
CH), 7.07–7.00 (m, 2H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.97–6.92 (m, 2H, Ar + CH
CH), 6.97–6.92 (m, 2H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.31 (br. s, 1H, OH), 3.68 (s, 3H, OMe), 3.60 (s, 3H, Me); 13C NMR (100 MHz, DMSO-d6) δ 181.9, 171.8, 165.8, 159.3, 159.0, 156.7, 156.6, 142.9, 140.9, 134.2, 133.4, 130.7, 129.0, 128.3, 126.4, 123.4, 122.8, 122.7, 116.4, 116.0, 114.9, 109.1, 106.1, 68.9, 55.2, 28.8; IR (NaCl, cm−1): 3456, 1839, 1728, 1662, 1639; MS: found 535.28; calcd for C31H23N2O7 (M + H)+ 535.15; EA: found C 69.78, H 4.02, N 5.23; calcd for C31H22N2O7 (534.52): C 69.66, H 4.15, N 5.24.
CH), 6.31 (br. s, 1H, OH), 3.68 (s, 3H, OMe), 3.60 (s, 3H, Me); 13C NMR (100 MHz, DMSO-d6) δ 181.9, 171.8, 165.8, 159.3, 159.0, 156.7, 156.6, 142.9, 140.9, 134.2, 133.4, 130.7, 129.0, 128.3, 126.4, 123.4, 122.8, 122.7, 116.4, 116.0, 114.9, 109.1, 106.1, 68.9, 55.2, 28.8; IR (NaCl, cm−1): 3456, 1839, 1728, 1662, 1639; MS: found 535.28; calcd for C31H23N2O7 (M + H)+ 535.15; EA: found C 69.78, H 4.02, N 5.23; calcd for C31H22N2O7 (534.52): C 69.66, H 4.15, N 5.24.
4′-Hydroxy-1′-(4-methoxyphenyl)-3′-(3-(4-methoxyphenyl)acryloyl)-5-methyl-2H-spiro[furo[3,2-c]quinoline-3,2′-pyrrole]-2,4,5′(1′H,5H)-trione (13cb)
Yield 435 mg (77%), mp 281–282 °C (dec.), colorless crystals. 1H NMR (400 MHz, DMSO-d6) δ 7.83–7.75 (m, 2H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.68–7.48 (m, 5H, Ar + CH
CH), 7.68–7.48 (m, 5H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.42–7.38 (m, 1H, Ar + CH
CH), 7.42–7.38 (m, 1H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.08–6.98 (m, 4H, Ar + CH
CH), 7.08–6.98 (m, 4H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.97–6.92 (m, 2H, Ar), 3.81 (s, 3H, OMe), 3.68 (s, 3H, OMe), 3.60 (s, 3H, Me); 13C NMR (100 MHz, DMSO-d6) δ 181.9, 171.8, 165.8, 161.5, 159.3, 159.2, 156.6, 155.9, 143.0, 140.9, 133.4, 130.3, 128.3, 126.8, 126.4, 122.8, 122.7, 120.9, 116.8, 115.9, 114.9, 114.6, 109.1, 106.1, 68.9, 55.3, 55.2, 28.8; IR (NaCl, cm−1): 3431, 1839, 1730, 1658, 1629; MS: found 565.27; calcd for C32H25N2O8 (M + H)+ 565.16; EA: found C 67.99, H 4.32, N 4.94; calcd for C32H24N2O8 (564.55): C 68.08, H 4.29, N 4.96.
CH), 6.97–6.92 (m, 2H, Ar), 3.81 (s, 3H, OMe), 3.68 (s, 3H, OMe), 3.60 (s, 3H, Me); 13C NMR (100 MHz, DMSO-d6) δ 181.9, 171.8, 165.8, 161.5, 159.3, 159.2, 156.6, 155.9, 143.0, 140.9, 133.4, 130.3, 128.3, 126.8, 126.4, 122.8, 122.7, 120.9, 116.8, 115.9, 114.9, 114.6, 109.1, 106.1, 68.9, 55.3, 55.2, 28.8; IR (NaCl, cm−1): 3431, 1839, 1730, 1658, 1629; MS: found 565.27; calcd for C32H25N2O8 (M + H)+ 565.16; EA: found C 67.99, H 4.32, N 4.94; calcd for C32H24N2O8 (564.55): C 68.08, H 4.29, N 4.96.
3′-Cinnamoyl-4′-hydroxy-1′-(4-methoxyphenyl)-5-phenyl-2H-spiro[furo[3,2-c]quinoline-3,2′-pyrrole]-2,4,5′(1′H,5H)-trione (13bc)
Yield 388 mg (65%), mp 290–291 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 7.88 (d, J = 7.9 Hz, 1H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.75–7.26 (m, 12H, Ar + CH
CH), 7.75–7.26 (m, 12H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.25–7.21 (m, 3H, Ar + CH
CH), 7.25–7.21 (m, 3H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.07 (d, J = 7.4 Hz, 1H, Ar + CH
CH), 7.07 (d, J = 7.4 Hz, 1H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.84 (d, J = 8.9 Hz, 2H, Ar + CH
CH), 6.84 (d, J = 8.9 Hz, 2H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.71 (d, J = 8.7 Hz, 1H, Ar + CH
CH), 6.71 (d, J = 8.7 Hz, 1H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.75 (s, 3H, OMe); 13C NMR (100 MHz, CDCl3) δ 183.4, 171.2, 166.0, 161.5, 160.3, 157.7, 144.7, 142.9, 136.7, 134.8, 132.9, 130.9, 130.4, 129.5, 129.3, 129.1, 129.1, 129.0, 128.9, 126.6, 123.7, 123.7, 123.1, 117.9, 117.0, 115.2, 110.4, 105.4, 70.1, 55.6; IR (NaCl, cm−1): 3423, 1839, 1716, 1667, 1639; MS: found 597.28; calcd for C36H25N2O7 (M + H)+ 597.17; EA: found C 72.39, H 4.01, N 4.76; calcd for C36H24N2O7 (596.60): C 72.48, H 4.06, N 4.70.
CH), 3.75 (s, 3H, OMe); 13C NMR (100 MHz, CDCl3) δ 183.4, 171.2, 166.0, 161.5, 160.3, 157.7, 144.7, 142.9, 136.7, 134.8, 132.9, 130.9, 130.4, 129.5, 129.3, 129.1, 129.1, 129.0, 128.9, 126.6, 123.7, 123.7, 123.1, 117.9, 117.0, 115.2, 110.4, 105.4, 70.1, 55.6; IR (NaCl, cm−1): 3423, 1839, 1716, 1667, 1639; MS: found 597.28; calcd for C36H25N2O7 (M + H)+ 597.17; EA: found C 72.39, H 4.01, N 4.76; calcd for C36H24N2O7 (596.60): C 72.48, H 4.06, N 4.70.
4′-Hydroxy-3′-(3-(4-methoxyphenyl)acryloyl)-5-phenyl-1′-(4-tolyl)-2H-spiro[furo[3,2-c]quinoline-3,2′-pyrrole]-2,4,5′(1′H, 5H)-trione (13dc)
Yield 440 mg (62%), mp 272–273 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 7.88 (dd, J = 8.0, 1.3 Hz, 1H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.72–7.36 (m, 8H, Ar + CH
CH), 7.72–7.36 (m, 8H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.29–7.09 (m, 6H, Ar + CH
CH), 7.29–7.09 (m, 6H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 7.05 (d, J = 7.7 Hz, 1H, Ar + CH
CH), 7.05 (d, J = 7.7 Hz, 1H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.87 (d, J = 8.8 Hz, 2H, Ar + CH
CH), 6.87 (d, J = 8.8 Hz, 2H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.70 (d, J = 8.6 Hz, 1H, Ar + CH
CH), 6.70 (d, J = 8.6 Hz, 1H, Ar + CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 3.83 (s, 3H, OMe), 2.29 (s, 3H, Me); 13C NMR (100 MHz, CDCl3) δ 183.2, 171.2, 166.0, 162.2, 161.4, 157.7, 144.7, 142.9, 139.6, 136.7, 132.8, 131.5, 130.8, 130.5, 130.4, 130.4, 129.4, 129.3, 128.9, 127.5, 127.4, 123.7, 123.0, 121.3, 117.0, 114.7, 110.4, 106.5, 70.0, 55.6, 21.3; IR (NaCl, cm−1): 3443, 1846, 1737, 1662, 1637; MS: found 611.33; calcd for C37H27N2O7 (M + H)+ 611.18; EA: found C 72.71, H 4.25, N 4.63; calcd for C37H26N2O7 (610.62): C 72.78, H 4.29, N 4.59.
CH), 3.83 (s, 3H, OMe), 2.29 (s, 3H, Me); 13C NMR (100 MHz, CDCl3) δ 183.2, 171.2, 166.0, 162.2, 161.4, 157.7, 144.7, 142.9, 139.6, 136.7, 132.8, 131.5, 130.8, 130.5, 130.4, 130.4, 129.4, 129.3, 128.9, 127.5, 127.4, 123.7, 123.0, 121.3, 117.0, 114.7, 110.4, 106.5, 70.0, 55.6, 21.3; IR (NaCl, cm−1): 3443, 1846, 1737, 1662, 1637; MS: found 611.33; calcd for C37H27N2O7 (M + H)+ 611.18; EA: found C 72.71, H 4.25, N 4.63; calcd for C37H26N2O7 (610.62): C 72.78, H 4.29, N 4.59.
Methyl 1′-benzyl-4′-hydroxy-2,4,5′-trioxo-1′,5′-dihydro-2H,4H-spiro[furo[3,2-c]chromene-3,2′-pyrrole]-3′-carboxylate (17aa)
Yield 359 mg (83%), mp 222–224 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 8.70 (br. s, 1H, OH), 7.74–7.65 (m, 2H, Ar), 7.45–7.33 (m, 2H, Ar), 7.14–7.06 (m, 5H, Ar), 4.49 (dd, J = 4.53, 4.70 Hz, 2H, CH2), 3.72 (s, 3H, OMe); 13C NMR (100 MHz, CDCl3) δ 168.9, 164.1, 164.0, 163.0, 157.8, 155.9, 155.1, 135.0, 133.0, 129.2, 128.8, 128.7, 125.1, 123.4, 117.7, 110.2, 108.2, 101.5, 65.6, 52.8, 46.0; IR (NaCl, cm−1): 3202, 1843, 1714, 1678, 1662, 1650; MS: found 434.15; calcd for C23H16NO8 (M + H)+ 434.09; EA: found C 63.71, H 3.46, N 3.28; calcd for: C23H15NO8 (433.37): C 63.75, H 3.49, N 3.23.Methyl 4′-hydroxy-2,4,5′-trioxo-1′-phenyl-1′,5′-dihydro-2H,4H-spiro[furo[3,2-c]chromene-3,2′-pyrrole]-3′-carboxylate (17ba)
Yield 352 mg (84%), mp 206–208 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 8.62 (br. s, 1H, OH), 7.72–7.65 (m, 2H, Ar), 7.43–7.27 (m, 5H, Ar), 7.24–7.19 (m, 2H, Ar), 3.79 (s, 3H, OMe); 13C NMR (100 MHz, CDCl3) δ 169.5, 164.7, 163.4, 163.1, 157.6, 156.0, 155.6, 135.1, 133.8, 130.2, 129.7, 127.6, 125.2, 123.6, 117.9, 110.1, 108.0, 101.1, 68.1, 52.9; IR (NaCl, cm−1): 3279, 1837, 1738, 1716, 1645; MS: found 420.14; calcd for C22H14NO8 (M + H)+ 420.07; EA: found C 62.91, H 3.06, N 3.38; calcd for C22H13NO8 (419.35): C 63.01; H 3.12; N 3.34.Methyl 4′-hydroxy-2,4,5′-trioxo-1′-(4-tolyl)-1′,5′-dihydro-2H,4H-spiro[furo[3,2-c]chromene-3,2′-pyrrole]-3′-carboxylate (17ca)
Yield 351 mg (81%), mp 210–212 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 7.71–7.64 (m, 2H, Ar), 7.43–7.33 (m, 2H, Ar), 7.17–7.07 (m, 4H, Ar), 6.30 (wide s, 1H, OH), 3.77 (s, 3H, OMe), 2.27 (s, 3H, Me); 13C NMR (100 MHz, CDCl3) δ 169.6, 164.7, 163.7, 163.1, 157.4, 156.0, 155.6, 140.0, 135.0, 130.9, 130.8, 127.4, 125.1, 123.7, 117.9, 110.1, 107.9, 101.2, 68.3, 52.8, 21.3; IR (NaCl, cm−1): 3148, 1852, 1743, 1722, 1692, 1652; MS: found 434.18; calcd for C23H16NO8 (M + H)+ 434.09; EA: found C 63.71, H 3.46, N 3.28; calcd for C23H15NO8 (433.37): C 63.75, H 3.49, N 3.23.Methyl 1′-benzyl-4′-hydroxy-2,4,5′-trioxo-5-phenyl-1′,4,5,5′-tetrahydro-2H-spiro[furo[3,2-c]quinoline-3,2′-pyrrole]-3′-carboxylate (17ac)
Yield 401 mg (79%), mp 244–246 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 8.72 (br. s, 1H, OH), 7.80 (dd, J = 8.0, 1.4 Hz, 1H, Ar), 7.62–7.44 (m, 4H, Ar), 7.31 (td, J = 7.7, 0.9 Hz, 1H, Ar), 7.22–7.05 (m, 7H, Ar), 6.74 (d, J = 8.6 Hz, 1H, Ar), 4.57 (s, 2H, CH2), 3.70 (s, 3H, OMe); 13C NMR (100 MHz, CDCl3) δ 170.3, 164.3, 163.2, 160.9, 157.7, 157.2, 142.9, 136.5, 134.2, 133.1, 130.4, 129.4, 129.5, 129.3, 129.1, 129.0, 128.6, 128.3, 123.3, 123.1, 117.0, 110.1, 108.6, 106.1, 66.6, 52.6, 45.9; IR (NaCl, cm−1): 3448, 1835, 1732, 1712, 1697, 1651, 1632; MS: found 509.28; calcd for C29H21N2O7 (M + H)+ 509.13; EA: found C 68.43, H 3.93, N 5.54; calcd for C29H20N2O7 (508.49): C 68.50, H 3.96, N 5.51.Methyl 4′-hydroxy-2,4,5′-trioxo-1′,5-diphenyl-1′,4,5,5′-tetrahydro-2H-spiro[furo[3,2-c]quinoline-3,2′-pyrrole]-3′-carboxylate (17bc)
Yield 341 mg (69%), mp 255–256 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 8.77 (br. s, 1H, OH), 7.81 (ddd, J = 8.0, 1.5, 0.5 Hz, 1H, Ar), 7.63–7.49 (m, 3H, Ar), 7.45 (ddd, J = 8.8, 6.6, 3.0 Hz, 1H, Ar), 7.36–7.23 (m, 7H, Ar), 7.03 (ddd, J = 6.4, 2.9, 1.5 Hz, 1H, Ar), 6.72 (d, J = 7.3 Hz, 1H, Ar), 3.77 (s, 3H, OMe); 13C NMR (100 MHz, CDCl3) δ 170.9, 163.7, 163.3, 161.2, 157.6, 157.4, 143.0, 136.5, 134.0, 133.2, 130.5, 130.5, 129.8, 129.5, 129.3, 129.2, 128.8, 127.7, 123.5, 123.2, 117.1, 110.0, 108.4, 105.9, 69.0, 52.7; IR (NaCl, cm−1): 3183, 1836, 1741, 1713, 1671, 1650, 1639; MS: found 495.22; calcd for C28H19N2O7 (M + H)+ 495.12; EA: found C 68.09, H 3.63, N 5.74; calcd for C28H18N2O7 (494.46): C 68.02, H 3.67, N 5.67.Methyl 4′-hydroxy-2,4,5′-trioxo-5-phenyl-1′-(4-tolyl)-1′,4,5,5′-tetrahydro-2H-spiro[furo[3,2-c]quinoline-3,2′-pyrrole]-3′-carboxylate (17cc)
Yield 325 mg (64%), mp 259–261 °C (dec.), colorless crystals. 1H NMR (400 MHz, CDCl3) δ 8.74 (wide s, 1H, OH), 7.81 (dd, J = 8.0, 1.3 Hz, 1H, Ar), 7.65–7.49 (m, 3H, Ar), 7.45 (ddd, J = 8.7, 7.2, 1.6 Hz, 1H, Ar), 7.34–7.22 (m, 2H, Ar), 7.18–7.01 (m, 5H, Ar), 6.74 (d, J = 8.6 Hz, 1H, Ar), 3.76 (s, 3H, OMe), 2.28 (s, 3H, Me); 13C NMR (100 MHz, CDCl3) δ 170.9, 163.8, 163.3, 161.1, 157.7, 157.5, 143.0, 139.5, 136.6, 133.1, 131.6, 130.5, 130.5, 129.6, 129.2, 128.8, 127.6, 123.5, 123.1, 117.1, 110.1, 108.3, 106.0, 69.0, 52.6, 21.3; IR: ν = 3188, 1840, 1733, 1708, 1650, 1639; MS: found 509.25; calcd for C29H21N2O7 (M + H)+ 509.13; EA: found C 68.59, H 3.91, N 5.53; calcd for C29H20N2O7 (508.49): C 68.50, H 3.96, N 5.51.Acknowledgements
Financial support for this work was provided by Ministry of Education and Science of the Russian Federation (project # 965).Notes and references
- (a) F. Medina, J. Marrero, M. Macias-Alonso, M. Gonzalez, I. Cordova-Guerrero, A. Garcia and S. Osegueda-Robles, Nat. Prod. Rep., 2015, 32, 1472 RSC; (b) L. Santana, E. Uriarte, F. Roleira, N. Milhazes and F. Borges, Curr. Med. Chem., 2004, 11, 3239 CrossRef CAS PubMed; (c) N. Lopez-Gutierrez, R. Romero-Gonzalez, J. Vidal, A. Frenich, L. Santana, E. Uriarte, F. Roleira, N. Milhazes and F. Borges, Food Chem., 2016, 201, 59 CrossRef CAS PubMed; (d) S. Ji, X. Qiao, Z. Li, Y. Wang, S. Yu, W. Liang, X. Lin and M. Ye, RSC Adv., 2015, 5, 45258 RSC; (e) S. Ji, W. Song, Y. Wang, W. Liang, K. Li, S. Tang, Q. Wang, X. Qiao, D. Zhou, S. Yu and M. Ye, J. Nat. Prod., 2016, 79, 281 CrossRef CAS PubMed; (f) M. Neacsu, N. Vaughan, V. Raikos, S. Multari, G. Duncan, G. Duthie and W. Russell, Food Chem., 2015, 179, 159 CrossRef CAS PubMed; (g) P. Pahari, U. Saikia, T. Das, C. Damodaran and J. Rohr, Tetrahedron, 2016, 72, 3324 CrossRef CAS; (h) N. Raghav, S. Jangra, A. Kumar, S. Bhattacharyya, D. Wadhwa and J. Sindhu, RSC Adv., 2016, 6, 34588 RSC; (i) T. Won, I. Song, K. Kim, W. Yang, S. Lee, D. Oh, W. Oh, K. Oh and J. Shin, J. Nat. Prod., 2015, 78, 666 CrossRef CAS PubMed; (j) G. Xi and Z. Liu, J. Agric. Food Chem., 2014, 62, 5636 CrossRef CAS PubMed; (k) Y. Yuan, Y. Feng, F. Ren, S. Niu, X. Liu and Y. Che, Org. Lett., 2013, 15, 6050 CrossRef CAS PubMed; (l) H. Morita, T. Dota and J. Kobayashi, Bioorg. Med. Chem. Lett., 2004, 14, 3665 CrossRef CAS PubMed; (m) M. Xu and Y. S. Kim, Food Chem. Toxicol., 2014, 74, 311 CrossRef CAS PubMed.
- (a) S. Hajdok, J. Conrad, H. Leutbecher, S. Strobel, T. Schleid and U. Beifuss, J. Org. Chem., 2009, 74, 7230 CrossRef CAS PubMed; (b) M. Rowley, J. Kulagowski, A. Watt, D. Rathbone, G. Stevenson, R. Carling, R. Baker, G. Marshall, J. Kemp, A. Foster, S. Grimwood, R. Hargreaves, C. Hurley, K. Saywell, M. Tricklebank and P. Leeson, J. Med. Chem., 1997, 40, 4053 CrossRef CAS PubMed; (c) Z. Xiao, N. Waters, C. Woodard, Z. Li and P. Lia, Bioorg. Med. Chem. Lett., 2001, 11, 2875 CrossRef CAS PubMed; (d) S. Angeleska, P. Kefalas and A. Detsi, Tetrahedron Lett., 2013, 54, 2325 CrossRef CAS; (e) F. O'Donnell, T. Smyth, V. Ramachandran and W. Smyth, Int. J. Antimicrob. Agents, 2010, 35, 30 CrossRef PubMed; (f) K. Kawashima, T. Inoue, N. Tsutsumi and H. Endo, Biochem. Pharmacol., 1996, 51, 139 CrossRef; (g) C. Moulis, K. R. Wirasutisna, J. Gleye, P. Loiseau, E. Stanislas and C. Moretti, Phytochemistry, 1983, 22, 2095 CrossRef CAS.
- See, for example: (a) Y. Tsuda, S. Hosoi, N. Katagari, C. Kaneko and T. Sano, Chem. Pharm. Bull., 1993, 41, 2087 CrossRef CAS; (b) Y. Tsuda, T. Ohshima, S. Hosoi, S. Kaneuchi, F. Kiuchi, T. Jun and T. Sano, Chem. Pharm. Bull., 1996, 44, 500 CrossRef CAS.
- (a) E. Ziegler, M. Eder, C. Belegratis and E. Prewedourakis, Monatsh. Chem., 1967, 98, 2249 CrossRef CAS; (b) G. Kollenz, H. Igel and E. Ziegler, Monatsh. Chem., 1972, 103, 450 CrossRef CAS; (c) B. Eistert, G. Muller and T. E. Arackal, Liebigs Ann. Chem., 1976, 1023 CrossRef CAS; (d) T. Sano, J. Toda and Y. Tsuda, Chem. Pharm. Bull., 1983, 31, 356 CrossRef CAS; (e) K. Isobe, C. Mohri, H. Sano, K. Mohri, H. Enomoto, T. Sano and Y. Tsuda, Chem. Pharm. Bull., 1989, 37, 3236 CrossRef CAS; (f) B. Eistert, G. Miiller and T. Arackal, Liebigs Ann. Chem., 1976, 1031 CrossRef CAS; (g) L. George, P. Bernhardt, K. Netsch and C. Wentrup, Org. Biomol. Chem., 2004, 2, 3518 RSC; (h) H. Abd El-Nabi, Tetrahedron, 2002, 58, 135 CrossRef CAS; (i) W. Fabian, J. Org. Chem., 2002, 67, 7475 CrossRef CAS PubMed.
- N. V. Bubnov, E. S. Denislamova, Z. G. Aliev and A. N. Maslivets, Russ. J. Org. Chem., 2011, 47, 526 CrossRef.
- (a) Y. N. Bannikova, E. A. Sedegova, V. V. Khalturina and A. N. Maslivets, Russ. J. Org. Chem., 2007, 43, 1338 CrossRef CAS; (b) E. S. Denislamova, A. Y. Dubovtsev, P. A. Slepukhin and A. N. Maslivets, Russ. J. Org. Chem., 2014, 50, 1017 CrossRef CASA. Y. Dubovtsev, E. S. Denislamova, M. V. Dmiriev and A. N. Maslivets, Russ. J. Org. Chem., 2016, 52, 706 CrossRef CAS.
- (a) Y. N. Bannikova, A. N. Maslivets, Y. S. Rozhkova, Y. V. Shklyaev and Z. G. Aliev, Mendeleev Commun., 2005, 15, 158 CrossRef; (b) E. S. Denislamova and A. N. Maslivets, Russ. J. Org. Chem., 2010, 46, 389 CrossRef CAS; (c) P. S. Silaichev, V. O. Filimonov, P. A. Slepukhin and A. N. Maslivets, Molecules, 2012, 17, 13787 CrossRef CAS PubMed.
- P. S. Silaichev, V. O. Filimonov, P. A. Slepukhin, M. Rubin and A. N. Maslivets, Eur. J. Org. Chem., 2015, 12, 2739 CrossRef.
- (a) Y. Tsuda, Y. Horiguchi and T. Sano, Heterocycles, 1976, 7, 1237 CrossRef; (b) V. O. Filimonov, P. A. Slepukhin and A. N. Maslivets, Russ. J. Org. Chem., 2012, 48, 561 CrossRef; (c) K. Mohri, A. Kanie, Y. Horiguchi and K. Isobe, Heterocycles, 1999, 51, 2377 CrossRef CAS.
- (a) D. Buckle, B. Cantello, H. Smith and B. Spicer, J. Med. Chem., 1975, 7, 726 CrossRef; (b) E. Ziegler and K. Gelfert, Monatsh. Chem., 1959, 6, 822 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, physico-chemical and spectral data. CCDC 1486439 and 1486440. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra16889b |
| This journal is © The Royal Society of Chemistry 2016 |