Multifunctional nano-engineered and bio-mimicking smart superhydrophobic reticulated ABS/fumed silica composite thin films with heat-sinking applications
Abstract
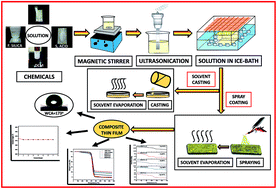
There are increasing requirements for engineered surfaces with distinct properties such as superhydrophobicity, self-cleaning, high thermal stability, and anti-corrosion. A number of materials for various applications have been created by adding fumed silica as a filler in polymeric materials. In the present work, we demonstrate that an engineering thermoplastic, acrylonitrile butadiene styrene (ABS), which has a high thermal stability and superior mechanical properties, can be ameliorated with nano fumed silica (FS) for applications requiring superhydrophobicity and heat resistance. An efficacious and cost effective combination of solvent casting and spray coating methods was used to prepare superhydrophobic thin films (0.1 mm thickness) of the ABS/FS composite. These composite thin films exhibited a superhydrophobic rough surface with a favourable water contact angle of 174° and an adequate resistance to UV irradiation. The reticulated superhydrophobic surface morphology of the ABS/FS composite thin films was confirmed by FE-SEM analysis. The water repellency of the superhydrophobic surfaces was demonstrated by the Wenzel, Cassie–Baxter, and McCarthy models. The elevated glass transition temperature (Tg) of 112 °C and the thermal stability of the thin films up to 200 °C, confirmed by DSC and TGA thermograms, respectively, make the ABS/FS composite a strong contender for heat-sinking applications in diverse electronic devices which have complex integrated circuits.

 Please wait while we load your content...
Please wait while we load your content...