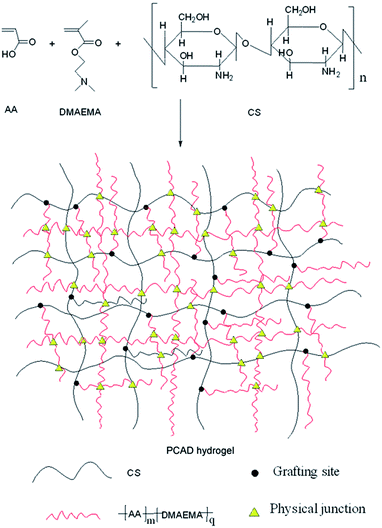
Physically cross-linked pH-responsive chitosan-based hydrogels with enhanced mechanical performance for controlled drug delivery
YuJu Chea,
Dongping Lia,
Yulong Liua,
Qinglin Maa,
Yebang Tanb,
Qinyan Yuec and
Fanjun Meng*a
aMarine College, Shandong University, Weihai 264209, PR China. E-mail: yjche@sdu.edu.cn; mengfj@sdu.edu.cn; Fax: +86 631 5688303; Tel: +86 631 5681086
bSchool of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, PR China
cSchool of Environmental Science and Engineering, Shandong University, Jinan 250100, PR China
First published on 31st October 2016
Abstract
A novel physically cross-linked pH-responsive hydrogel with enhanced mechanical performance (PCAD) was prepared from chitosan (CS), acrylic acid (AA) and (2-dimethylamino) ethyl methacrylate (DMAEMA) via in situ free radical polymerization for controlled drug delivery. The successful fabrication of the hydrogels was verified by Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) measurements. Scanning electron microscopy (SEM) and mechanical analyses demonstrated that the morphological and mechanical behaviors of the resultant hydrogels were strongly affected by the content of AA and DMAEMA. Moreover, the swelling properties of these hydrogels were systematically investigated, and the results indicated that they exhibited strong pH sensitivity. The drug delivery applications of such fabricated hydrogels were further evaluated, from which Bovine serum albumin (BSA) and 5-fluorouracil (5-Fu) were chosen as the model drugs for in vitro release. The results showed that the amount of 5-Fu and BSA released can be tuned by changing the composition of the hydrogel and the pH of the medium. Toxicity assays confirmed that the blank hydrogels had negligible toxicity to normal cells, whereas the 5-Fu-loaded hydrogels remained high in cytotoxicity for LO2 and HepG-2 cancer cells. As seen from the results, PCAD hydrogels seem to have a potential application in drug-delivery systems controlled by the external pH value for cancer therapy.
Introduction
Hydrogels are three dimensional crosslinked hydrophilic polymeric networks that do not dissolve in water but can imbibe significant amounts of water or other biological fluids and exhibit swelling behavior at physiological conditions (pH and temperature).1 They have been extensively explored because of their many properties such as high water content, good biocompatibility, soft consistency similar to natural tissue, structural integrity, and elasticity, resulting in a wide range of applications in various fields including drug delivery,2,3 tissue engineering,4 pharmaceutics,5 sensors,6 actuators,7 contact lenses,8 water purification,9 regenerative medicine,10 textiles,11 and so on. Recently, stimuli-responsive hydrogels which can undergo reversible changes in response to environmental stimuli such as temperature,12 pH,13 solvent composition,14 light,15 and magnetic field,16 have attracted great interests due to their particular applications in biomedical applications, such as controlled drug release, enzyme immobilization, separation, biosensors, microfluidics and tissue engineering.17 Among all of the above mentioned stimuli, pH responsive hydrogels have been extensively investigated for potential use in site-specific delivery of drugs to specific regions of the gastrointestinal tract and have been prepared for delivery of different dimensional drugs, ranging from protein to peptide to small molecular drugs, because pH is most often changed in a physiological, biological and chemical systems.18 They can be applied as inserts or implants or can be administered orally, subcutaneously or intramuscularly. They maintain a sustained level of drug in blood stream and also protect drugs against enzymatic degradation and have good patient compliance.19,20 In the recent past, many pH responsive hydrogels have been synthesized and investigated under different aspects by many researchers, including hydrogels for anticancer drug delivery which accelerated the anticancer drug release under acidic conditions,3,20–24 hydrogels for oral protein drug delivery which were pH responsive to protect proteins from the harsh stomach environment, mucoadhesive to prolong the hydrogel residence time in the intestine,19,25–28 and hydrogels for colon-targeted drug delivery which delivered drugs efficiently to the colon with little or no release of the drug in the upper gastrointestinal tract (GIT).29–32Mostly investigated pH-responsive polymer gel networks are commonly prepared from poly(acrylic/methacrylic acid) PAA/PMAA, sulphonic acid and sulphonamide based polymers, in particular PAA.33,34 It swells in basic pH solutions due to the formation of carboxylate anions and collapses in acidic pH solutions because the carboxylic groups are protonated and unionized. However, extensive swelling of uncross-linked PAA in water limits its applications in drug delivery because of its dissolution before delivery of drug.35 Generally, the PAA hydrogels are prepared with organic cross-linkers, such as N,N-methylenebisacrylamide (BIS), tripropylene glycol diacrylate, etc., which are usually toxic, and the organic cross-linker gels are often brittle and inextensible due to their poor toughness limiting their potential applications.36 Enormous efforts have been made to develop a new material that could overcome these disadvantages. For example, hydrogels made by combining biocompatible PAA with natural or semi-synthetic biopolymers based on polysaccharides or proteins have been widely explored to achieve high concentration of drugs in the specific region or tissue and the controlled release profile for extended time periods.37 Chitosan (CS), including its derivatives, as a widely-studied biopolymer, is one of the most abundant naturally occurring polysaccharide, which has attracted much interest in the biomedical fields such as drug-release carriers and tissue engineering because of its excellent biodegradability, biocompatibility, antimicrobial activity and mucoadhesive properties.38,39 Low molecular weight chitosan (≤50 kDa), instead of high molecular weight chitosan (≥100 kDa), has been suggested as promising excipients for drug delivery in cases in which additional physicochemical properties in the polymer structure are desirable. It has been shown that low molecular weight chitosan showed higher biodegradable, biological activity and gene transfection efficiency.40–43 The hydrogels made from CS and PAA with no chemical cross-linkers have been reported by many researchers and widely used for study of drug release. Shim et al. carried out template polymerization of acrylic acid in the presence of CS by gamma radiation and this hydrogel was reported for release of 5-fluorouracil drug.44 Torre et al. prepared hydrogel of CS and PAA by blending these two polymers in aqueous acetic acid solution followed by freeze drying to produce the network and amoxicillin drug was incorporated in situ in the blend network.45 In these studies hydrogels were prepared either by blending PAA and CS or polymerizing acrylic acid in the presence of CS by gamma radiations, whose stability of these gels depends on freeze drying and formation of polyion complexes between cationic CS (due to its NH3+ group) and anionic PAA. Thus, their pH response rate is slow and their mechanical properties are poor which greatly restrict their applications.
In the past few decades 5-fluorouracil (5-Fu), which is a sparingly water soluble pyrimidine antimetabolite and is having short biological half-life of 10–20 min resulting from the incomplete and nonuniform oral absorption due to rapid metabolism caused by dihydro pyrimidine dehydrogenase, has been employed as one of the potential antitumor drugs in clinical chemotherapy specifically for the treatment of solid tumors, including colorectal cancer.46–48 Due to the poorly tumor selective, the therapeutic use of 5-Fu leads to cause toxicity to healthy cells, which therefore results in toxic side effects of bone marrow, gastro intestinal tract and central nervous system.24,30,32,49 To reduce these side effects and as well as to improve the antitumor activity, many researchers have been investigated on pH responsive hydrogels for controlled delivery of 5-Fu. EI-Sherbiny et al. developed pH responsive IPN hydrogel form poly(N-acryloylglycine-chitosan) for controlled release of 5-Fu at 37 °C in buffer solutions at pH 2.1 and 7.4. The in vitro release profiles of 5-Fu from the gels shown that release at pH 2.1 is greater when compared to pH 7.4.50 Qian et al. prepared pH-thermo dual sensitive hydrogels via inclusion complexation with α-cyclodextrin and the random copolymers poly[N,N-dimethylaminoethyl methacrylate-co-poly(poly(ethylene glycol) methyl ether methacrylate)] for controlled release application. These hydrogels released considerably more 5-Fu in pH 2.0 than pH 7.4.21 Anirudhan et al. developed pH responsive composite hydrogel by grafting β-cyclodextrin to gelatin and crosslinking with oxidised dextran and investigated the colon delivery of 5-Fu under both simulated gastric (pH 1.2) and intestinal (pH 7.4) conditions. These gels released almost 85% of the loaded drug within 24 h in pH 7.4 buffer, but pH 1.2 it released about 50% of the loaded drug in 24 h.30 Rao et al. prepared pH responsive hydrogels composed of 2-(dimethylamino) ethylmethacrylate and 2-hydroxyethylacrylate via free-radical polymerization at 29 °C for controlled delivery of 5-Fu to treat colon cancer. The in vitro release data indicated that the maximum drug release was significantly achieved in pH 1.2 rather than in pH 7.4 and it was enhanced up to 30 h.24
The aim of this work was to develop a novel pH-responsive hydrogel based on chitosan with enhanced mechanical performance (PCAD), which was fabricated via in situ free radical polymerization of acrylic acid (AA) and (2-dimethylamino) ethyl methacrylate (DMAEMA) in acetic acid solution of CS without any chemical cross-linkers for site-specific delivery of drugs to specific regions of the gastrointestinal tract. In the past few years, due to their stimuli-responsive properties, plenty of hydrogels synthesized by DMAEMA are synthesized for broad application in controlled drug delivery,21,24,27 water treatment,51 etc. Since it can be protonized in acid condition, DMAEMA was a pH-responsive monomer. Thus incorporation of DMAEMA functionality to the hydrogels of CS and PAA may create stronger physical cross-linking through intra- and inter-chain association via non-covalent forces, such as hydrogen bonding, hydrophobic staking and electrostatic interaction,52,53 endowing the hydrogels with more pH sensitive and excellent mechanical properties. Furthermore, it is known that DMAEMA was an accelerator of copolymerization reaction due to possible redox reaction between tertiary amino groups of DMAEMA and potassium persulfate, which speed the gelation even at room temperature.54 The swelling behavior of the hydrogel PCAD was investigated as function of pH, salt concentration, content of AA and DMAEMA. The rheological and mechanical properties were investigated. In addition, cytotoxicity of the hydrogel PCAD was studied, and the release profiles of bovine serum albumin (BSA) and 5-fluorouracil (5-Fu) from the hydrogel PCAD were investigated.
Experimental
Materials
Chitosan (CS, weight-average molecular weight = 50 kDa and with a deacetylation of 87%) was purchased from Qingdao Hecreat Bio-tech Company Ltd. Acrylic acid (AA, 99%) and 2-(dimethylamino)ethyl methacrylate (DMAEMA, 98%) were purchased from J&K Chemical Ltd. and were distilled prior to use. Bovine serum albumin (BSA, 99%) and Bradford reagent were purchased from J&K Chemical Ltd. 5-Fluorouracil (5-Fu, 98%) was purchased from Heowns Biochem LLC and used as received. Potassium persulfate (98%) and sodium thiosulfate (98%) were from Kermel Chemical Ltd. and used without any further purification. DMEM (phenol red-free medium) containing 10% (v/v) fetal bovine serum (FBS) and 10 mg mL−1 ciprofloxacin, thiazolyl blue tetrazolium bromide (MTT) and dimethylsulfoxide (DMSO) were obtained from Sigma-Aldrich. HepG-2 and LO2 cell lines were supplied by Sigma-Aldrich. Deionized water was used in all experiments unless otherwise specified.Preparation of PCAD hydrogels
PCAD hydrogels with different composition were synthesized via in situ free radical copolymerization of AA and DMAEMA in CS acetic acid solution as shown in Table 1. In a typical polymerization, firstly 0.1 g CS was added into 10 mL acetic acid solution (1 wt%) in a glass beaker and dissolved completely under stirring after about 6 h. Then AA and DMAEMA was mixed thoroughly at 0 °C in a two-necked round bottom flask for about 5 min. Potassium persulfate (0.015 g) and Sodium thiosulfate (0.015 g) were dissolved in 1 mL deionized water. Then, the three solutions were mixed in a test tube (internal diameter 3 mm and length 80 mm) along with constant nitrogen purging for about 15 minutes and sonicated for another 2 minutes to expel the entrapped air. Afterward, the test tube was sealed with Teflon tape and the polymerization was carried out for 24 h at 50 °C. After the polymerization, the copolymer hydrogel, which was cut into small pieces, was immersed in deionized water for three consecutive days to remove the unreacted monomers, during which water was replaced every 4 hours on the first day and twice a day for the remaining period. The hydrogels were dried to a constant weight by lyophilize and then stored in a vacuum desiccator for further used.| Sample code | AA (mass %) | DMAEMA (mass %) | Monomer conversiona (%) | Tg (°C) | Tdec (°C) | Char yield (%) |
|---|---|---|---|---|---|---|
| a Monomer conversion was determined using gravimetric method by comparing the weight of purified dry gel with respect to the weight of monomer in feed. | ||||||
| PCAD0 | 100 | 0 | 97 | — | — | — |
| PCAD1 | 97.5 | 2.5 | 97 | 77.1 | 233.5 | 14.45 |
| PCAD2 | 95 | 5 | 98 | 78.4 | 244.3 | 11.83 |
| PCAD3 | 90 | 10 | 99 | 80.3 | 246.2 | 10.04 |
| PCAD4 | 70 | 30 | 98 | 84.5 | 250.8 | 7.123 |
Characterization of the PCAD hydrogels
FTIR spectra of PACD hydrogels were recorded on a FTIR spectrometer (Vertex70, Bruker Co, Germany) using KBr pellet made by mixing KBr with fine powder of the gel samples. X-ray diffraction (XRD) patterns of the samples were obtained with a Rigaku Ultima IV X-ray diffractometer using CuKα radiation with a wavelength of 0.154 nm at a voltage of 340 kV and a current of 20 mA over the range of 4–50 °C. SEM images were recorded with a Nova Nano SEM NPEP281 was observed by using a scanning electron microscope (SEM). The samples were equilibrated in deionized water at room temperature for at least 24 h, and then refrigerated for more than 24 h and dried using vacuum freezing and sputter-coated with PdAu before imaging. The differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) measurements were carried out on a Mettlertoledo TGA/DSC/1600LF Scanning calorimetry (Switzerland) in nitrogen atmosphere at the scanning rate of 10 °C min−1 in the temperature range of 30–600 °C. Compression tests were performed with a Universal Testing Machine (Instron 5967) at room temperature. Before testing, cylinder-shaped gel samples were equilibrated in distilled water at room temperature (25 °C) for 24 h. Then, the swollen gel samples were cut into pieces (diameter of 15.5 mm, thickness of about 7.0 mm). A load cell of 1000 N was used to compress the samples up to the required strain and the test speed was 1 mm min−1. The rheological properties of PCAD hydrogels were determined using an Haake Mars III rheometer using an oscillatory testing mode. A 26 mm parallel plate–Peltier plate geometry and a gap of 1 mm were used for all assays which were done at 25 °C. To ensure the measurements were performed in the linear region of viscoelasticity, an amplitude sweep was carried out, and the results displayed no variation in elastic modulus up to a strain of 1.0%. The dynamic modulus of the PCAD hydrogels was recorded as a frequency function, where the frequency sweeps were measured between 0.1 and 10 Hz. The tests were repeated three times, and the results were averaged.Swelling behavior
Swelling ratios (SRs) of hydrogels were gravimetrically measured after removing excessive surface water with wet filter paper in the pH range from 1.2 to 8.0. SR is calculated using the following equation:
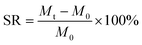 | (1) |
Drug loading and in vitro drug release behavior
5-Fu and BSA were chosen as the model drugs and loaded to the as-prepared hydrogels using the swelling diffusion method. A certain weight of dried hydrogel was immersed in 50 mL PBS solution (pH 7.4) containing specified amount (m0) of 5-Fu or BSA drug at 37 °C for 48 h, and then refrigerated for more than 24 h and dried using vacuum freezing to get a drug-loaded hydrogel. The drug loading (DL) and entrapment efficiency of the hydrogel was calculated using the following equation:
 | (2) |
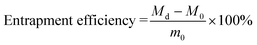 | (3) |
In vitro release of the drugs from the hydrogel samples was carried out at 37 °C at a rotation speed of 100 rpm in 50 mL of two different pH (1.2 and 7.4) PBS buffer solutions in order to mimic the gastro intestinal conditions. The dry drug-loaded samples were immersed in buffer solution of same composition. At several time intervals 2 mL of the solution containing released drug was withdrawn and at the same time 2 mL fresh solution was added to keep the solution volume constant. The concentration of drug in the withdrawn solution was analyzed by UV-visible spectrophotometer (Hitachi, U-2910) at a wavelength of 262 nm for 5-Fu or by MICRO SCANTM MS5608 microplate reader (ECIL, India) using Bradford method at a wavelength of 595 nm for BSA. All release experiments were carried out in triplicates and the average values were considered. The accumulative percent drug release (Er) was obtained using the following equation:
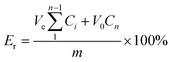 | (4) |
Cell toxicity
The cytotoxicity of the PCAD hydrogels and 5-Fu loaded PCAD hydrogels were assessed by MTT assay with HepG-2 (lung cancer cells) and LO2 cells (lung cells). First, HepG-2 and LO2 were seeded at 1 × 105 cells per well in a 96-well plate (corning, USA) in DMEM with 10% FBS and ciprofloxacin (10 mg mL−1), which was maintained at 37 °C and 5% CO2 in humidified atmosphere.The 5-Fu-free hydrogels were sterilized with ultraviolet ray lamp for 15 min followed by rinsing with sterile pH 7.4 PBS. Then, the sterile hydrogels were added into 5 mL of DMEM at 37 °C for 48 h. Subsequently, the hydrogels were carefully taken out, and the resulting extract solutions were filtrated through a 0.22 μm syringe filter. Additionally, the 5-Fu-loaded hydrogels were immersed in DMEM for 48 h and then taken out from the buffer. After that, free 5-Fu solution and the extract liquid were sterilized by filtration (0.22 μm) and diluted with DMEM culture media (the concentration of 5-Fu in extract solution was the same as that of free 5-Fu, ultimate concentration 2.5, 5, 10, 20, 40 μg mL−1).
For the MTT assay, the cells were seeded into 96-well plates at densities of 1 × 105 cells per well. After the incubation with different concentrations of the samples (5-Fu-free hydrogel extracts, free 5-fluorouracil, or 5-fluorouracil-loaded hydrogel extract solution) for 72 h, the cells were washed with PBS and processed for MTT assay to determine the cell viability as previously detailed.55 Cell viability was determined by the absorbance values at 570 nm which was measured with a microplate reader (Versa max).
Results and discussion
Synthesis and characterization of PCAD hydrogels
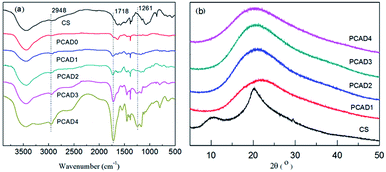
The main objective of this study was to prepare high pH-responsive PCAD hydrogels that have excellent mechanical properties via in situ free radical polymerization of acrylic acid (AA) and (2-dimethylamino) ethyl methacrylate (DMAEMA) in acetic acid solution of CS to form a semi-interpenetrating polymer network (semi-IPN), whose synthesis scheme is shown in Fig. 1. All gelation reactions were carried out for 24 h to reach the maximum monomer conversion. After purification, yield of PCAD hydrogels was determined by gravimetric method with respect to weight of monomers in feed and results are shown in Table 1. In our experiment, when DMAEMA is absent or 50 wt% in the recipe, the polymerization products is too soft to maintain a certain shape in water, while when DMAEMA is fed with 70 wt%, the resulting product is soluble in water and no hydrogel can be obtained. In view of practical applications, DMAEMA is increased from 2.5 to 30 wt% to prepare hydrogels with higher mechanical strength. As expected, PCAD hydrogels become tougher with increasing DMAEMA content, which is supported by mechanical tests discussed later. On the other hand, the result reflects that the structure of PCAD hydrogels can be controlled by varying the DMAEMA feed ration, as proved by the following SEM observations. PCAD hydrogels show excellent pH-responsive properties which were then used as triggering mechanisms for controlled release of 5-Fu and BSA model drugs.To investigate the structural aspects of PCAD hydrogels, FTIR spectra of the gels listed in Table 1 were recorded, which were shown in Fig. 2a. As compared to the spectra of CS and PCAD0 hydrogel, PCAD1–4 hydrogels exhibited the characteristic absorption signals of carboxylic acid groups of AA: 1462 cm−1 (the symmetrical stretch vibration of –CO–OH) and 1681 cm−1 (the overlapping of the asymmetrical stretch vibration of –COOH and the deforming vibration of –NH2 of CS), and the signals of functional groups of DMAEMA: 1261 cm−1 (the stretching vibration of –C–N), 1718 cm−1 (the stretching vibration of the ester group –OOC–) and 2948 cm−1 (the stretching vibration of the bands of methyl groups that are adjacent to the nitrogen atom). Meanwhile, it is evident that the strength of these absorption becomes stronger in the PCAD1–4 hydrogels when the feed ratio of DMAEMA increases, which indicates that the content of DMAEMA segments in PCAD hydrogels can be controlled by the feed ratio.
XRD was used to characterize further the microstructures of CS and PCAD hydrogels, as shown in Fig. 2b. The XRD pattern of CS shows two main characteristic diffraction peaks at (2θ) 11.4° and 20.2°, which are typical for the semi-crystal structure of CS.56 The PCAD hydrogel diffraction patterns have only one broad amorphous diffraction peaks at (2θ) 20.0° corresponding to the amorphous P(AA-co-DMAEMA) matrix and no distinct diffraction peak at around (2θ) 11.4°, which indicates that no significant CS aggregation phenomenon occurs in PCAD hydrogels.
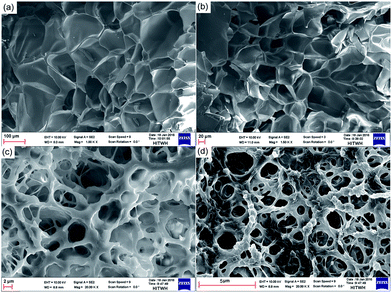
The morphological characteristics of PCAD hydrogels after exposure to solutions and subsequent freeze drying have been examined by SEM, and obtained micrographs are shown in Fig. 3. The porous structure of PCAD hydrogel is obvious, and the micrographs clearly illustrate that PCAD hydrogel morphology is dependent on the DMAEMA content. PCAD 1 hydrogel (Fig. 3a) has the largest average pore size and a relatively uniform wall thickness of pores. For PCAD 4 (Fig. 3d) hydrogel, its pore size decreases while the pore density increases. Increasing the DMAEMA feed ratio, the interior structure of PCAD hydrogel becomes inhomogeneous, manifested by its uneven pore size and wall thickness. From PCAD 1 to PCAD 4 hydrogels, their average pore sizes are around 112.5, 63.45, 19.8 ± 3 and 7.65 μm respectively. This result indicates that the structure uniformity and the average pore size of PCAD hydrogels decrease under higher degree of cross-linking that is determined by the DMAEMA feed ratio.57 So, it can be concluded that the swelling ratio decreased with an increasing content of DMAEMA.
Thermal properties
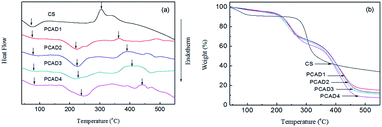
The thermal properties of CS and PCAD hydrogels were investigated using DSC and TGA, which is shown in Fig. 4 and the related data are summarized in Table 1. As shown in Fig. 4a, the thermogram of CS exhibits two distinct stages. The one in the range 30–100 °C peaking at 72 °C is correspond to its glass transition temperature Tg, and the other in the range 230–360 °C with maximum decomposition rate at 303 °C has been ascribed to a complex process including dehydration of the saccharide rings, depolymerization and decomposition of the acetylated and deacetylated units of the polymer.58,59 As for PCAD hydrogels, the Tg for PCAD1, PCAD2, PCAD3 and PCAD4 are 77.1, 78.4, 80.3 and 84.5 °C, respectively, increasing as the DMAEMA content increased. The reason is probably because the degree of cross-linking of PCAD hydrogels became higher with more DMAEMA content, which results in a decrease in the free volume between the polymer chains, as shown by SEM observations. When the temperature is above Tg, two main decomposition stages are observed. The first one starts at 170 °C peaking at 222–253 °C depending on the sample, which is mainly due to the dehydration and decarboxylation reactions of P(AA-co-DMAEMA) as normal polymers do60,61 and the deacetylation of CS moieties.62 It is worth mentioning, however, that the maximum decomposition rate in this stage is more than 45 °C lower than that of CS, indicating unstabilization of the polymer as a result of complexation. This phenomenon has also been reported by other reporters.63,64 The second degradation stage of PCAD hydrogels starts at about 342 °C and exhibits a maximum degradation rate at approximately 375–440 °C depending on the sample, which corresponds mainly to the degradation of the P(AA-co-DMAEMA) chains and the rest of the CS backbone. It can be seen that the temperatures at which the maximum decomposition rate is in those two stages increases regularly when the DMAEMA feed ratio changes from 2.5 to 30 wt% (Table 1), further indicating the control of actual DMAEMA content in PCAD hydrogels by feed ratio.The TGA curves of CS and PCAD hydrogels are shown in Fig. 4b. The initial CS exhibits two distinct weight losses in its thermogravimetric curve. The one in the range of 30–100 °C, peaking at 72.6 °C, is associated with loss of water (4.5 wt%), and the real thermal degradation starts from the second step of weight loss in the range 230–360 °C, with maximum decomposition rate at 300 °C, has been ascribed above. For PCAD hydrogels, there are mainly two steps of weight loss in TGA curves. The first step from 169 °C to 300 °C is due to the dehydration and decarboxylation of P(AA-co-DMAEMA) and deacetylation of CS moieties, releasing water, ammonia and small quantities of carbon dioxide. At temperatures above 320 °C, bulk decomposition of P(AA-co-DMAEMA) main chain and CS residue take place, releasing carbon dioxide, water, nitrile compounds and imides.65 Additionally, as listed in Table 1, the decomposition temperatures (Tdec) of PCAD1, PCAD2, PCAD3 and PCAD4 are 233.5, 244.3, 246.2 and 250.8 °C respectively, and the char yield of PCAD hydrogels decrease regularly from 14.45 to 7.12 wt% with DMAEMA content increasing, which were in consistence with the results of DSC curve.
Mechanical properties
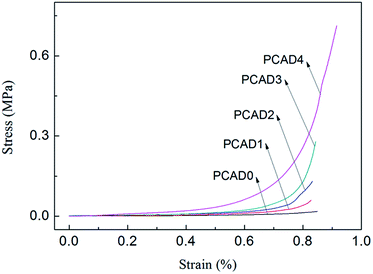
It is known that mechanical properties of hydrogels are very important for pharmaceutical applications. A drug delivery system designed to protect a sensitive therapeutic agent (such as protein) must maintain its integrity to be able to protect the protein until it is released out of the system,66 and it is important to have the ability to modify the structural and mechanical properties of the hydrogel in a controlled manner.67 Compression test was carried out to study the mechanical properties of PCAD hydrogels, as shown in Fig. 5 and Table 2. From Fig. 5, it can be observed that no failure or damage occurs in the compression process of PCAD hydrogels. As compared with PCAD0, the compressibility of PCAD1–4 hydrogels has been dramatically improved. For instance, when the strain is 85%, the compressive strength of PCAD0 is 17.92 kPa, while the compressive strength of PCAD4 hydrogel with 30 wt% DMAEMA achieved at about 0.41 MPa, increased by about 2188% compared to PCAD0 hydrogel. It can be seen from Table 2 that the PCAD hydrogels showed higher compressive strength and modulus than those in the previous studies through various chemical and physical bonds for enhancing the mechanical properties.55,68 Of course, it is only an approximate comparison, since the parameters regarding characterization of compressive properties of hydrogels differed. Moreover, it was observed that the mechanical properties of PCAD hydrogels strongly depend on the contents of DMAEMA. The compressive modulus which was calculated as a slope from the linear part of the stress–strain curve (strain from 0% to 30%) and the strength at the same strain e.g. of 80% of PCAD hydrogels increased with increasing DMAEMA feed ratios. This can be well explained by the higher degree of cross-linking with higher DMAEMA content, resulting in smaller pore size and increased pore density as observed through SEM analysis (Fig. 3). Pore size is an important factor governing mechanical strength, as in case of matrices with smaller pore size, the load gets distributed evenly throughout the surface. Well-connected small pores form a barrier and avert catastrophic crack propagation.27,69Mechanical strength of PCAD hydrogels in equilibrium swollen condition has also been investigated by rheology. Measurements of storage modulus (G′) and loss modulus (G′′) versus % strain have been carried out with swelled PCAD hydrogels in order to find out the linear viscoelastic regime (Fig. 6a and b) and a linear viscoelastic regime up to 3% has been observed. Therefore frequency sweep experiment of swelled PCAD hydrogels has been carried out at a constant strain of 1%, which is well below the deformation limit (3%) of PCAD hydrogels. As indicated in Fig. 5c and d, both G′ and G′′ of PCAD hydrogels changed little with increasing oscillation frequency (from 0.1 to 10 Hz). All PCAD hydrogels exhibited very high G′ than G′′ throughout the whole frequency range, indicating quite sable viscoelastic solid-like behavior which is a prominent elastic property of strong gels, due to the three-dimensional network structure.70,71 With the increase of DMAEMA content in PCAD hydrogels, the G′ increases from 75 to 946 Pa. This observation proved that mechanical property of chitosan-based PAA hydrogels can be improved by copolymerization with DMAEMA comonomer, which was consistent with the previously reported findings indicating that the higher content of DMAEMA induced a higher intermolecular cross-linking within the hydrogels and created a more compact network structure, thus leading to a greater stiffness.27
 | ||
| Fig. 6 Rheological properties of PCAD hydrogels: (a) G′-strain curves; (b) G′′-strain curves; (c) G′-frequency curves; (d) G′′-frequency curves. | ||
Swelling behaviors
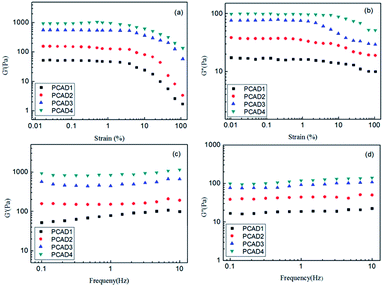
The capacity of swelling is one of the most important parameters to evaluate the properties of hydrogels. Swelling kinetics profiles of PCAD hydrogels were investigated in pH 7.4 neutral medium (distilled water at 25 °C) in order to avoid influence of ions that can make up the buffer solution and results at a consecutive time intervals was plotted in Fig. 7a. It was found that all the lyophilized PCAD hydrogels revealed a rapid swelling rate in the first 10 h of immersion and achieved equilibration by 50 h of incubation. The equilibrium swelling ratios (ESR) of PCAD1, PCAD2.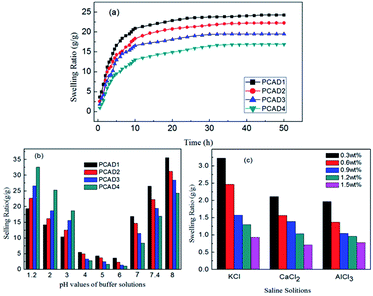 | ||
| Fig. 7 (a) Swelling ratio values in deionized water; (b) swelling kinetics in different buffer solutions; and (c) salt solutions. | ||
PCAD3 and PCAD4 hydrogels were 26.53, 22.29, 19.47 and 16.92, respectively. That is, the ESR values of different PCAD hydrogels decreases in the order of PCAD1 > PCAD2 > PCAD3 > PCAD4 as DMAEMA content increases in the hydrogels, due to the increase of cross-linking density. The swelling ratio of a polymeric hydrogel is also inversely proportional to the mechanical strength. This conclusion about the swelling ratios and the mechanical strength for the PCAD1, PCAD2, PCAD3 and PCAD4 hydrogels is consistent with the results of compression test and rheological test.
To investigate the influence of pH value of the medium on the swelling ratios for the PCAD hydrogels, the pH range is selected from 1.2 to 8 in this study. The ionic strength in various buffer media was adjusted to be 0.1mol L−1 by NaCl. As shown in Fig. 7b, the swelling ratios of all the PCAD hydrogels gradually decreased steadily and reached minimal values at a certain pH, and then increased again with an increase of pH value. This phenomenon may be attributed to the ionization behavior of the amine group of DMAEMA (pKa ≈ 2.5) and carboxylic acid side group of AA (pKa ≈ 4.5) repeating units in response to external pH changes. The amine group of DMAEMA repeating units becomes protonated at low pH, which increased the charge density of the hydrogel. The hydrogel become more hydrophilic and the repulsive force between cationic DMAEMA repeat units expands the spaces of the networks to retain more water, so they become swollen. When the pH value increased, the percentage of ionic DMAEMA repeat units decreases, the inter-/intramolecular hydrogen bonding between the free amino groups of DMAEMA repeat units and the carboxylic acid groups of AA repeat units become dominant in the polymer network, which causes the observed decrease of the swelling ratio of the hydrogels. The swelling ratios of PCAD hydrogels decreased to the lowest level when the pH value increases to 4–6. With further increase of the pH values, the swelling ratios of PCAD hydrogels increase again. This is because as the pH value of the medium increases, the carboxylic acid groups become ionized, most of the hydrogen bonding are broken and the electrostatic repulsion between the molecular chains is predominated which leads to the network more expanding. The result also revealed a dependence of the swelling ratio on the content of DMAEMA in the resulting hydrogels. While the swelling ratio of the hydrogels increased with increasing the content of the DMAEMA at lower pH value (<4), which may be attributed to the presence of more positively charged amino groups, leading to an expansion in the hydrogel network, the swelling ratios of the hydrogels decreased with increasing the content of the DMAEMA at higher pH value (>5), which is due to the presence of smaller negatively charged carboxylic acid groups of AA repeat units. Furthermore, the pH value at which the swelling ratio is lowest of PCAD1, PCAD2, PCAD3 and PCAD4 was found to be 4.1, 4.6, 5.1 and 5.6, respectively, which increases with the increment of DMAEMA content in the hydrogels. This pH-sensitive swelling behavior is of significant importance in controlled delivery of drugs in medium with different pH, which makes PCAD hydrogels as suitable candidate for designing drug delivery systems.
The swelling behaviors of the hydrogel are considered as an important factor, influencing the drug releasing rate and the cumulative drug release. PCAD hydrogels are ionized hydrogels that their swelling behavior depends on both the characteristics of the chemical structure and the medium. Generally, the extent to that the hydrogel swells at equilibrium increases with more functional ionizable groups on the network and decreases with higher extent of cross-linking occurred during the synthetic step(s). It is well known that the swelling of ionic hydrogels in saline solutions was appreciably decreased compared to the values measured in deionized water, which is often attributed to a charge screening effect of the additional cations causing a non-perfect anion–anion electrostatic repulsion, leading to a decreased osmotic pressure (ionic pressure) difference between the hydrogel network and the external solution.72 Considering the practical application of hydrogel, the study on swelling behavior of PCAD hydrogels in salt solution seems to be more significant. In this investigation, the swelling behaviour of the PCAD4 hydrogel in different salt solutions was studied and present in Fig. 7c. It is clear that the swelling ratios of PCAD4 hydrogel were significantly affected by salt concentrations of the surrounding medium. Regardless of salt species, an increase in ionic strength causes a significant decrease in the swelling ratios. The similar effect of salt concentration on the swelling behavior also has been observed in other polyelectrolyte hydrogel systems.56,73 Generally, swelling property of polyelectrolyte hydrogels is governed by the osmotic imbalance generated due to the free exchange of mobile ions and the water molecules between the gel matrix and the external solution and as well as due to the electrostatic repulsion between the similar fixed charges connected with the polymer chain within the gel network. The mechanisms of this phenomenon can be understood as follows. On the one hand, the osmotic pressure between the external incubation medium and the interior hydrogel was strengthened with the increment of saline solution concentration. In this case, water in the hydrogel network tended to diffuse out to the swelling solution, which had a high salt concentration, leading to acceleration of water molecules release and reduction of swelling capacity. On the other hand, the counterions (such as K+) penetrated into the inside structure of the hydrogel and combined with carboxylic groups (–COO−) of AA repeat units, causing the shielding effect and weakening the anion–anion electrostatic repulsion. Hence, the moistures were squeezed out from the gel. Furthermore, it is also exhibited in this graph that the swelling ratios strongly depended on ionic valence of the introducing salt. Apparently, at a given salt concentration, the swelling of the hydrogel was trivalent Al3+ < divalent Ca2+ < monovalent K+. This is because that the high charge cations (e.g., Ca2+ and Al3+) could induce the formation of intra- and intermolecular complexes with anionic groups (–COO−) on the AA repeat units via electrostatic interaction, which augmented the cross-linking density of the hydrogel and gave rise to a dense structure, limiting the environmental solution entry into the network of the hydrogels, thus leading to a remarkable decrement of ESR with increase of the metal cationic valence of the salt solution.
In vitro drug release study
As PCAD hydrogels had relatively high water swell-ability and showed good responses to the changes of pH, they were selected as representative samples for controlled drug release studies. The loading and entrapment efficiency of 5-Fu and BSA drug for various hydrogels are shown in Table 3. Similar to swelling ratio, loading and entrapment efficiency of these drugs are also observed to decrease with increase in content of DMAEMA, due to the increase of cross-linking density. The loading or entrapment efficiency of BSA is observed to be higher than loading or entrapment of 5-Fu, this is because that while 5-Fu is a negatively charged drug,74 BSA is an ampholyte with both amido and carboxyl groups. At pH 7.4, most of carboxyl groups of AA repeat units in PCAD hydrogels were ionized to anion groups (–COO−), so the stronger anion–anion repulsive forces between 5-Fu and PCAD hydrogels led to the lower drug loading or entrapment efficiency of 5-Fu compared to that of BSA. The cumulative release profiles of 5-Fu (Fig. 8a and b) and BSA (Fig. 8c and d) from these hydrogels at pH 1.2 and 7.4 are presented in Fig. 8. Form all of these figures it is observed that an initial burst release of the drug is followed by a sustained rate of release for all of these hydrogels. Initially the fast release rate of drug occurs from the surface of the hydrogel due to high concentration gradient of the drug between the release medium, i.e., water and the surface of the gel.75 As the release of the drug continues, its concentration in the release medium increases and hence the concentration gradient of drug between gel and release medium decreases and entrapment of the remaining drug in the gel network slow down further release at low concentration gradient.56,75 It is also observed that BSA shows slightly lower release% than 5-Fu at both pH 1.2 and 7.4. That is, at pH 1.2 in 10 h, about 15.42%, 25.31%, 35.62% and 46.1% of 5-Fu were released from PCAD1, PCAD2, PCAD3 and PCAD4, respectively, and about 9.76%, 17.09%, 25.16% and 35.81% of BSA were released from PCAD1, PCAD2, PCAD3 and PCAD4, respectively. When the pH is 7.4, in 10 h, for 5-Fu it is about 78.82%, 61.41%, 46.42% and 27.31% were released from PCAD1, PCAD2, PCAD3 and PCAD4, respectively, while for BSA, it is about 71.12%, 43.4%, 29.6% and 20.71% were released from PCAD1, PCAD2, PCAD3 and PCAD4, respectively. In general, the drug release character from hydrogel is related to the swelling behaviors of polymeric hydrogel in the medium. Besides, the various interactions between drug molecules and polymeric network also play a key role in the drug release behaviors.76 At pH 7.4, as discussed above, more rapid release of 5-Fu is due to the stronger anion–anion repulsive forces with PCAD hydrogels compared to BSA which has a stronger hydrogen bonding interactions with PCAD hydrogels. At pH 1.2, most amino groups of both DMAEMA repeat units in PCAD hydrogels and BSA are positively charged, leading to stronger cation–cation repulsive forces between BSA and PCAD hydrogels and stronger electrostatic attraction between 5-Fu and PCAD hydrogels. Consequently, the cumulative release% of BSA should be faster than that of 5-Fu. However, 5-Fu appears to be structurally more compact and smaller than BSA which may cause its easier diffusion out the gel. Furthermore, both 5-Fu and BSA release depend upon the amount of DMAEMA in PCAD hydrogels as well as the pH of the medium. It could be seen that the cumulative release% of both 5-Fu and BSA at pH 1.2 increased in the following order: PCAD1 < PCAD2 < PCAD3 < PCAD4. But at pH 7.4, the cumulative release% pattern of both 5-Fu and BSA is in the reverse order i.e. it followed the order PCAD1 > PCAD2 > PCAD3 > PCAD4. These results agreed well with the water swelling behavior of the PCAD hydrogels. According to Fig. 7b, with the increment of DMAEMA content, the swelling ratio of PCAD hydrogel increases at acidic solution but decreased in the neutral and alkaline solution. The higher swelling ratio of PCAD hydrogel created larger surface areas and porous aperture for the drug release. Consequently, the bigger aperture led to a high-drug release because of the lower hindrance in drug releasing. Thus the amount of 5-Fu and BSA released can be tuned by changing the composition of the hydrogel and the pH of the medium. As already said above, the release rates of both 5-Fu and BSA from PCAD1, PCAD2 and PCAD3 hydrogels were slow at pH 1.2 (15.42%, 25.31%, 35.62% cumulative release of 5-Fu from PCAD1, PCAD2 and PCAD3, respectively, in 10 h; 9.76%, 17.09%, 25.16% cumulative release of BSA from PCAD1, PCAD2 and PCAD3, respectively, in 10 h) and much faster at pH 7.4 (78.82%, 61.41%, 46.42% cumulative release of 5-Fu from PCAD1, PCAD2 and PCAD3, respectively, in 10 h; 71.12%, 43.4%, 29.6% cumulative release of BSA from PCAD1, PCAD2 and PCAD3, respectively, in 10 h), and this support the potential of the hydrogel as vehicle for targeted drug delivery in colon. However for PCAD4 hydrogel, both 5-Fu and BSA are more rapidly released at pH 1.2 (46.1% cumulative release of 5-Fu, 35.81% cumulative release of BSA, in 10 h) than at pH 7.4 (27.31%% cumulative release of 5-Fu, 20.71% cumulative release of BSA, in 10 h). Such pH-sensitive release behavior of PCAD4 hydrogel can protect the drug at high pH and release the drug at low pH, which would be desirable for cancer treatment, since tumor tissues are known to be acidic.23 Due to the composition of PCAD hydrogels, release results obtained in acidic and neutral pH values are very different. Drug release values can be lower or higher at acidic (or basic) pHs. This is an important observation in terms of the release studies performed both acidic and neutral media. So, PCAD hydrogels can be suitable for the application in wide pH range for the drug release at targeted site.| Sample code | 5-Fu/BSA loading (mg g−1 hydrogel sample) | 5-Fu/BSA entrapment efficiency (%) |
|---|---|---|
| PCAD1 | 29.3/382.3 | 58.6/76.4 |
| PCAD2 | 23.6/293.4 | 47.3/69.2 |
| PCAD3 | 15.8/221.4 | 31.5/55.8 |
| PCAD4 | 11.4/166.9 | 22.8/43.2 |
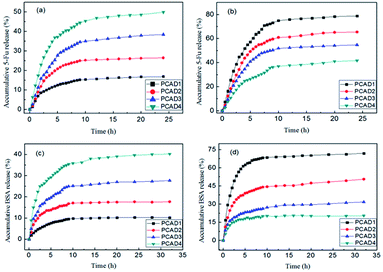 | ||
| Fig. 8 In vitro drug release behaviors from the PCAD hydrogels: (a) 5-Fu at pH 1.2; (b) 5-Fu at pH 7.4; (c) BSA at pH 1.2; (d) BSA at pH 7.4. | ||
Cytotoxicity assay
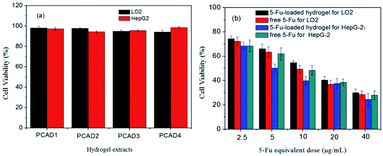
Cytotoxicity of biomaterials is an important aspect for its future application. To act as a safe and efficient drug carrier, the vector itself was required to be nontoxic to the normal cells, and the drug loaded vehicle should cause high cytotoxicity to tumor cells. Herein, the cytotoxicity of drug-free PCAD hydrogels to normal cell lines LO2 was first investigated by an indirect MTT assay. As presented in Fig. 9a, the viabilities of LO2 cells cultured with different extracts of empty hydrogel samples were all above 90% after 72 h of incubation, which demonstrated that these hydrogels were biocompatible with negligible cytotoxicity at concentrations up to 200 mg mL−1 and could be utilized as a delivery platform. Further, we screened the cytotoxic effect of the blank hydrogel in HepG2 cancer cell lines. It also can be seen from Fig. 9a that the hydrogel alone did not exhibit any appreciable cytotoxicity to HepG2 cancer cells.In addition, the antitumor activity of 5-Fu-loaded hydrogels (PCAD4) against LO2 cells and HepG2 cells was also estimated via the MTT method, and the free 5-Fu was employed as a control (Fig. 9b). After a 72 h of exposure, 5-Fu-loaded hydrogels showed a dose-dependent cell proliferation inhibition behavior for either LO2 cells or HepG2 cells, which was similar to that of free 5-Fu, revealing that the cytotoxicity was caused by released 5-Fu and not by the hydrogel system itself. More attractive, 5-Fu-loaded hydrogels exhibited lower cell growth inhibition efficiency for LO2 cells but a higher cell growth inhibition efficiency for HepG2 cells as compared with pure 5-Fu at the equivalent drug concentration. For instance, the cell viability of free 5-Fu (5 μg mL−1) and 5-Fu-loaded hydrogels (at an equivalent 5-Fu concentration of 5 μg mL−1) was 63.47% and 66.21% for LO2 cells. This fact might be ascribed to the delayed but sustained release of 5-Fu from the 5-Fu-loaded hydrogels and the decline in cellular uptake of 5-Fu within 72 h. However, the cell viability of free 5-Fu (5 μg mL−1) and 5-Fu-loaded hydrogels (at an equivalent 5-Fu concentration of 5 μg mL−1) was 61.93% and 50.23% for HepG2 cells. That is, the 5-Fu-loaded hydrogel exhibited superior cytotoxicity against HepG2 cells, indicating that at the same treatment dose 5-Fu-loaded hydrogels exhibited more antitumor activity compared with free 5-Fu. These finding shows that 5-Fu-loaded hydrogel can be better uptake by cancer cells and used as an intriguing vehicles for application in the field of targeted drug delivery.
Conclusions
In this paper, a series of stable, physically cross-linked hydrogels with tunable mechanical properties, pH-sensitive and swell ability were prepared by single step aqueous copolymerization of CS, AA and DMAEMA. The resultant hydrogels were characterized in detail. By adjusting the concentration of AA and DMAEMA used in the synthetic process, the properties of the fabricated hydrogels were precisely controlled, including mechanical behaviors, swelling properties and drug release behaviors. Additionally, the prepared hydrogel has pH and salinity sensitivities. The swelling ratio of the hydrogel can be tailored by external pH and salinity changes, and the sustained 5-Fu and BSA release from the hydrogel could also be modulated by varying the pH of the release media. Moreover, the in vitro cytotoxicity studies verified that the hydrogels display excellent compatibility with cells, and 5-Fu-loaded hydrogels exhibited lower cell growth inhibition efficiency for normal LO2 cells but a higher cell growth inhibition efficiency for cancer HepG2 cells as compared with pure 5-Fu at the equivalent drug concentration. With these excellent features, we can foresee that this CS-based pH-responsive hydrogel holds great promise to be utilized as a vehicle for anticancer drug delivery.Acknowledgements
The authors gratefully acknowledge the financial supports from the Major Research of Science and Technology, China (Grant No. 2011ZX05024-04-008) and Marine Institute of Shandong University (Weihai).References
- B. V. Slaughter, S. S. Khurshid, O. Z. Fisher, A. Khademhosseini and N. A. Peppas, Adv. Mater., 2009, 21, 3307–3329 CrossRef CAS.
- E. J. Cho, B. Sun, K.-O. Doh, E. M. Wilson, S. Torregrosa-Allen, B. D. Elzey and Y. Yeo, Biomaterials, 2015, 37, 312–319 CrossRef CAS PubMed.
- X. Qi, W. Wei, J. Li, G. Zuo, X. Hu, J. Zhang and W. Dong, RSC Adv., 2016, 6, 69869–69881 RSC.
- X.-L. He, L.-L. Ge, Z.-L. Liu, W.-J. Lu, J.-Q. Li, Y.-M. Zhao, X.-X. Li, N. Yang, L. Chen and D.-S. Wei, Ind. Eng. Chem. Res., 2014, 53, 10618–10628 CrossRef CAS.
- N. Peppas, P. Bures, W. Leobandung and H. Ichikawa, Eur. J. Pharm. Biopharm., 2000, 50, 27–46 CrossRef CAS PubMed.
- J. H. Owino, O. A. Arotiba, P. G. Baker, A. Guiseppi-Elie and E. I. Iwuoha, React. Funct. Polym., 2008, 68, 1239–1244 CrossRef CAS.
- M. E. Harmon, M. Tang and C. W. Frank, Polymer, 2003, 44, 4547–4556 CrossRef CAS.
- L. Xinming, C. Yingde, A. W. Lloyd, S. V. Mikhalovsky, S. R. Sandeman, C. A. Howel and L. Liewen, Cont. Lens Anterior Eye, 2008, 31, 57–64 CrossRef PubMed.
- D. Xu, S. Hein, L. S. Loo and K. Wang, Ind. Eng. Chem. Res., 2011, 50, 6343–6346 CrossRef CAS.
- K. Pal, A. Banthia and D. Majumdar, Des. Monomers Polym., 2009, 12, 197–220 CrossRef CAS.
- B. Liu and Y. Hu, Fibres Text. East. Eur., 2005, 13, 45–49 CAS.
- N. Rasool, T. Yasin, J. Y. Y. Heng and Z. Akhter, Polymer, 2010, 51, 1687–1693 CrossRef CAS.
- L. Li, J. Gu, J. Zhang, Z. Xie, Y. Lu, L. Shen, Q. Dong and Y. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 8033–8040 CAS.
- E. Kokufata, Y.-Q. Zhang and T. Tanaka, Nature, 1991, 351, 302–304 CrossRef CAS.
- J. Wu, A. Chen, M. Qin, R. Huang, G. Zhang, B. Xue, J. Wei, Y. Li, Y. Cao and W. Wang, Nanoscale, 2015, 7, 1655–1660 RSC.
- S.-J. Park, H.-S. Lim, Y. M. Lee and K.-D. Suh, RSC Adv., 2015, 5, 10081–10088 RSC.
- T. R. Hoare and D. S. Kohane, Polymer, 2008, 49, 1993–2007 CrossRef CAS.
- J. Zhao, X. Zhao, B. Guo and P. X. Ma, Biomacromolecules, 2014, 15, 3246–3252 CrossRef CAS PubMed.
- N. Rasool, T. Yasin, J. Y. Heng and Z. Akhter, Polymer, 2010, 51, 1687–1693 CrossRef CAS.
- X. Hu, Y. Wang, L. Zhang, M. Xu, W. Dong and J. Zhang, Carbohydr. Polym., 2017, 155, 242–251 CrossRef CAS PubMed.
- M. Zhou, K. Liu and X. Qian, J. Appl. Polym. Sci., 2016, 133, 43279–43285 Search PubMed.
- X. Qi, W. Wei, J. Li, Y. Liu, X. Hu, J. Zhang, L. Bi and W. Dong, ACS Biomater. Sci. Eng., 2015, 1, 1287–1299 CrossRef CAS.
- N. Gao, S. Lü, C. Gao, X. Wang, X. Xu, X. Bai, C. Feng and M. Liu, Chem. Eng. J., 2016, 287, 20–29 CrossRef CAS.
- S. Eswaramma, K. Krishna Rao and K. Madhusudana Rao, Int. J. Polym. Mater. Polym. Biomater., 2016, 65, 134–142 CrossRef CAS.
- K. Chaturvedi, K. Ganguly, M. N. Nadagouda and T. M. Aminabhavi, J. Controlled Release, 2013, 165, 129–138 CrossRef CAS.
- A. Das, M. Mehndiratta, P. Chattopadhyay and A. R. Ray, J. Appl. Polym. Sci., 2010, 115, 393–403 CrossRef CAS.
- D. Suhag, R. Bhatia, S. Das, A. Shakeel, A. Ghosh, A. Singh, O. Sinha, S. Chakrabarti and M. Mukherjee, RSC Adv., 2015, 5, 53963–53972 RSC.
- N. Ahmad, M. C. I. M. Amin, S. M. Mahali, I. Ismail and V. T. G. Chuang, Mol. Pharmaceutics, 2014, 11, 4130–4142 CrossRef CAS.
- R. K. Mishra and A. R. Ray, J. Appl. Polym. Sci., 2011, 119, 3199–3206 CrossRef CAS.
- T. S. Anirudhan and A. M. Mohan, RSC Adv., 2014, 4, 12109–12118 RSC.
- M. U. Minhas, M. Ahmad, J. Anwar and S. Khan, Adv. Polym. Technol., 2016, 21659–21667 Search PubMed.
- R. Mishra, K. Ramasamy and A. Majeed, J. Appl. Polym. Sci., 2012, 126, E98–E107 CrossRef CAS.
- A. Pourjavadi, M. Ayyari and M. S. Amini-Fazl, Eur. Polym. J., 2008, 44, 1209–1216 CrossRef CAS.
- J. A. Reddy and P. S. Low, J. Controlled Release, 2000, 64, 27–37 CrossRef CAS.
- X. Jin and Y.-L. Hsieh, Polymer, 2005, 46, 5149–5160 CrossRef CAS.
- T. Shimizu, M. Yamato, A. Kikuchi and T. Okano, Biomaterials, 2003, 24, 2309–2316 CrossRef CAS PubMed.
- A. Bertz, S. Wöhl-Bruhn, S. Miethe, B. Tiersch, J. Koetz, M. Hust, H. Bunjes and H. Menzel, J. Biotechnol., 2013, 163, 243–249 CrossRef CAS PubMed.
- I. M. El-Sherbiny, R. J. Lins, E. M. Abdel-Bary and D. R. K. Harding, Eur. Polym. J., 2005, 41, 2584–2591 CrossRef CAS.
- V. Dodane and V. D. Vilivalam, Pharm. Sci. Technol. Today, 1998, 1, 246–253 CrossRef CAS.
- M. Thanou, M. Nihot, M. Jansen, J. C. Verhoef and H. Junginger, J. Pharm. Sci., 2001, 90, 38–46 CrossRef CAS PubMed.
- S. Mao, X. Shuai, F. Unger, M. Simon, D. Bi and T. Kissel, Int. J. Pharm., 2004, 281, 45–54 CrossRef CAS PubMed.
- A. Vila, A. Sánchez, K. Janes, I. Behrens, T. Kissel, J. L. V. Jato and M. J. Alonso, Eur. J. Pharm. Biopharm., 2004, 57, 123–131 CrossRef CAS PubMed.
- V. E. Tikhonov, E. A. Stepnova, V. G. Babak, I. A. Yamskov, J. Palma-Guerrero, H.-B. Jansson, L. V. Lopez-Llorca, J. Salinas, D. V. Gerasimenko and I. D. Avdienko, Carbohydr. Polym., 2006, 64, 66–72 CrossRef CAS.
- J. W. Shim and Y. C. Nho, J. Appl. Polym. Sci., 2003, 90, 3270–3277 CrossRef CAS.
- P. M. D. L. Torre, Y. Enobakhare, G. Torrado and S. Torrado, Biomaterials, 2003, 24, 1499–1506 CrossRef PubMed.
- K. Chaturvedi, A. R. Kulkarni and T. M. Aminabhavi, Ind. Eng. Chem. Res., 2011, 50, 10414–10423 CrossRef CAS.
- B. Y. Swamy, J. H. Chang, H. Ahn, W.-K. Lee and I. Chung, Cellulose, 2013, 20, 1261–1273 CrossRef CAS.
- Z.-Y. Tian, G.-J. Du, S.-Q. Xie, J. Zhao, W.-Y. Gao and C.-J. Wang, Molecules, 2007, 12, 2450–2457 CrossRef CAS PubMed.
- N. S. Reddy and K. K. Rao, Indian J. Adv. Chem. Sci., 2016, 4, 214–234 Search PubMed.
- I. El-Sherbiny, R. Lins, E. Abdel-Bary and D. Harding, Eur. Polym. J., 2005, 41, 2584–2591 CrossRef CAS.
- B. Wang, X.-D. Xu, Z.-C. Wang, S.-X. Cheng, X.-Z. Zhang and R.-X. Zhuo, Colloids Surf., B, 2008, 64, 34–41 CrossRef CAS.
- K. M. Huh, H. C. Kang, Y. J. Lee and Y. H. Bae, Macromol. Res., 2012, 20, 224–233 CrossRef CAS.
- H. Mori and T. Endo, Macromol. Rapid Commun., 2012, 33, 1090–1107 CrossRef CAS.
- H. Li, R. Wu, J. Zhu, P. Guo, W. Ren, S. Xu and J. Wang, J. Polym. Sci., Part B: Polym. Phys., 2015, 53, 876–884 CrossRef CAS.
- X. Hu, L. Feng, A. Xie, W. Wei, S. Wang, J. Zhang and W. Dong, J. Mater. Chem. B, 2014, 2, 3646–3658 RSC.
- H. S. Samanta and S. K. Ray, Carbohydr. Polym., 2014, 106, 109–120 CrossRef CAS.
- L. Weng, A. Gouldstone, Y. Wu and W. Chen, Biomaterials, 2008, 29, 2153–2163 CrossRef CAS.
- C. Peniche-Covas, W. Argüelles-Monal and J. San Román, Polym. Degrad. Stab., 1993, 39, 21–28 CrossRef CAS.
- J. G. Alonso, C. Peniche-Covas and J. M. Nieto, J. Therm. Anal., 1983, 28, 189–193 CrossRef.
- Z. Xiaojing, L. Chong, H. Yuelei, L. Ruixue, H. Linghao and F. Shaoming, Polym. Int., 2014, 63, 2030–2041 CrossRef.
- Z. Özbaş and G. Gürdağ, J. Appl. Polym. Sci., 2015, 132, 41886–41896 CrossRef.
- I. C. McNeill and S. M. T. Sadeghi, Polym. Degrad. Stab., 1990, 30, 213–230 CrossRef CAS.
- C. Peniche, W. Argüelles-Monal, N. Davidenko, R. Sastre, A. Gallardo and J. San Román, Biomaterials, 1999, 20, 1869–1878 CrossRef CAS PubMed.
- W. Argüelles-Monal, M. Gárciga and C. Peniche-Covas, Polym. Bull., 1990, 23, 307–313 CrossRef.
- S. Shekhar, M. Mukherjee and A. K. Sen, Iran. Polym. J., 2012, 21, 895–905 CrossRef CAS.
- N. A. Peppas, P. Bures, W. Leobandung and H. Ichikawa, Eur. J. Pharm. Biopharm., 2000, 50, 27–46 CrossRef CAS PubMed.
- R. M. Jane, A. Plumb and S. B. Kaye, Cancer Res., 1989, 49, 4435–4440 Search PubMed.
- F. Mirahmadi, M. Tafazzoli-Shadpour, M. A. Shokrgozar and S. Bonakdar, Mater. Sci. Eng. C, 2013, 33, 4786–4794 CrossRef CAS PubMed.
- B. B. Mandal, S. Kapoor and S. C. Kundu, Biomaterials, 2009, 30, 2826–2836 CrossRef CAS PubMed.
- P. Khoshakhlagh and M. J. Moore, Acta Biomater., 2015, 16, 23–34 CrossRef CAS PubMed.
- A. K. Jha, R. A. Hule, T. Jiao, S. S. Teller, R. J. Clifton, R. L. Duncan, D. J. Pochan and X. Jia, Macromolecules, 2009, 42, 537–546 CrossRef CAS PubMed.
- D. Castel, A. Ricard and R. Audebert, J. Appl. Polym. Sci., 1990, 39, 11–29 CrossRef CAS.
- X. Qi, W. Wei, J. Li, Y. Liu, X. Hu, J. Zhang, L. Bi and W. Dong, ACS Biomater. Sci. Eng., 2015, 1, 1287–1299 CrossRef CAS.
- E. van den Bosch and C. Gielens, Int. J. Biol. Macromol., 2003, 32, 129–138 CrossRef CAS PubMed.
- D.-Q. Wu, Y.-X. Sun, X.-D. Xu, S.-X. Cheng, X.-Z. Zhang and R.-X. Zhuo, Biomacromolecules, 2008, 9, 1155–1162 CrossRef CAS PubMed.
- D. Kuilin, T. Hua, Z. Pengfei, Z. Haibin, R. Xiaobo and W. Haijun, J. Appl. Polym. Sci., 2009, 114, 176–184 CrossRef.
| This journal is © The Royal Society of Chemistry 2016 |