Development of functionalized multi-walled carbon nanotube-based polysaccharide–hydroxyapatite scaffolds for bone tissue engineering†
R. Rajeshab,
Y. Dominic Ravichandran*a,
M. Jeevan Kumar Reddyc,
Sung Hun Ryuc and
A. M. Shanmugharaj*c
aDepartment of Science and Humanities, Karpagam College of Engineering, Coimbatore-641032, India. E-mail: ydominic64@yahoo.co.in; Tel: +91-9443-898-857
bMaterials Chemistry Division, School of Advanced Sciences, VIT University, Vellore-632014, Tamil Nadu, India
cDepartment of Chemical Engineering, Kyung Hee University, Yongin, Kyunggi-Do 446701, South Korea. E-mail: shanmughar@gmail.com
First published on 15th August 2016
Abstract
New tricomponent composite scaffolds with high porosity (82.82% for oxidized carbon nanotube (fMWCNT)–gellan–hydroxyapatite (HAP) and 91.76%for fMWCNT–amylopectin–HAP) were prepared by a freeze drying method and characterized by Fourier transform infrared spectroscopy (FT-IR), X-ray diffraction (XRD), thermogravimetric analysis (TGA) and field emission scanning electron microscopy (FE-SEM). Further, their ability to promote cell proliferation of the MG 63 cell line for bone regeneration was studied. In vitro MTT assays with the MG 63 cell line demonstrated better cell proliferation on both the fMWCNT–gellan–HAP and fMWCNT–amylopectin–HAP scaffolds. The cell adhesion of the MG 63 cells on the scaffolds after four days was confirmed by fluorescence imaging using Hoechst and Acridine Orange dye staining. Enhanced mineralization over time was observed in the alkaline phosphatase (ALP) activity. A higher compressive strength (222.60 ± 8.4 kPa) was observed for fMWCNT–gellan–HAP, when compared to fMWCNT–amylopectin–HAP (57.32 ± 2.4 kPa). This may be due to the lower porosity of fMWCNT–gellan–HAP. These results ascertained that the prepared tricomponent scaffolds can be used as promising materials in bone tissue engineering.
Introduction
Development of biomaterials for bone engineering applications has gained major attention due to several medical applications including reconstruction of large orthopedic defects. Even though traditional bone graft techniques like allografts and autografts have been used to treat bony defects, disease transmission, the viability of the host bed and the size of the defect can limit their application.1–5 Metal implants used as an alternative for bone grafts also have several disadvantages like poor integration with the tissue at their implantation site which can eventually lead to failure or secondary surgery. Further, ceramic implants exhibit limitations of low tensile strength and brittleness.6 Hence, the development of 3D scaffolds with required porosity can support new tissue regeneration or replacement in a natural way and thereby can act as alternative solutions for the existing limitations of bone tissue engineering.1,5,7 Bone engineering typically involves an artificial extra-cellular matrix (ECM) (or scaffold), and osteoblasts that can regulate factors and promote cell recruitment, cell growth, differentiation and mineralization of bone tissue formation. Among them, highly porous scaffolds have the ability to accommodate osteoblasts or osteoprogenitor cells, to support cell proliferation and differentiation thereby playing a critical role in new tissue formation.8 Isolation of specific cells through a small biopsy from the patient and growing them on a three-dimensional (3D) scaffold under controlled culture conditions is a common approach. Subsequently, the construct is delivered to the desired site in the body of the patient with an aim to promote new tissue formation. An alternative approach is to implant scaffolds for tissue in-growth directly for the purpose of stimulating tissue formation in situ; this has the advantage of reducing the number of operations required, resulting in a shorter recovery time for the patients.9Contemporary biomaterials for bone tissue regeneration are a combination of inorganic and organic materials that mimic natural bone.7,10 HAP has been used as an inorganic material because of its biocompatibility and similarities in structure and composition to the inorganic elements of human bone.5,7,11 Carbonated HAP isolated from natural sources possesses higher biocompatibility than synthesized HAP.2,12 Hence, natural HAP isolated from chicken bones12 is used in this study. The brittle nature of HAP makes it unfit for load bearing applications in bone tissue engineering. A polysaccharide is incorporated with natural HAP to overcome its brittleness and to biologically mimic the role of the natural ECM.7,11,13–16
Gellan gum, an anionic tetrasaccharide, composed of a repeating unit sequence of β-D-glucose, β-D-glucuronic acid, β-D-glucose and α-L-rhamnose is normally produced by the bacterium Pseudomonas elodea, is non toxic and resembles the ECM glycosaminoglycan. Hence, it can be used as an additive in pharmaceutical applications.17,18 Further, amylopectin is another branched polysaccharide which is normally present in plants, and is composed of 1,4-linked α-D-glucose units with shorter branched 1,6-linked α-D-glucose units.19 It exhibits excellent pore forming ability, an antibacterial nature, good cell proliferation and bone formation.20 The bioactivity and biocompatibility of gellan gum and amylopectin along with the above mentioned properties have made them promising components in the fabrication of 3D scaffolds.
To increase the mechanical strength of this combination of HAP and polysaccharides, carbon nanotubes (CNTs) were incorporated as a third component.3,21–23 Even though there are many reports about the cytotoxicity of CNTs, there are also reports in the literature indicating the biocompatibility of functionalized CNTs for orthopaedic applications.15,24 More importantly, the geometry of CNTs resembles triple collagen fibrils present in natural bone.25
There are many CNT based tricomponent composites for bone engineering including multi walled carbon nanotube (MWCNT)–chitosan–HAP,26 MWCNT–gelatin–HAP,27 MWCNT–polypropylene (PP)–HAP,28 CNT–poly(caprolactone)–HAP29 and MWCNT–poly(L-lactic acid)–HAP30 and dicomponent composites including gellan gum–HAP and single walled carbon nanotube (SWCNT)–amylopectin. To our knowledge, there are no reports on the in vitro studies and mechanical strength of the above mentioned dicomponent composites.19,31 Further, gellan gum and amylopectin based tricomponent composite scaffolds with fMWCNTs and HAP have not been explored so far. Hence, the focus of the present work is to investigate the physicochemical, morphological, mechanical and biological properties of new CNT based tricomponent scaffolds of fMWCNT–gellan–HAP and fMWCNT–amylopectin–HAP for bone tissue engineering.
Results and discussion
Chemical composition and preparation of the scaffolds
The chemical composition of the scaffold was chosen to be similar to that of natural bone (70% HAP, 29% polysaccharide and 1% fMWCNTs). In addition to the chemical composition, the 3D structure and porosity have to be considered for the development of scaffolds in tissue engineering. The presence of porosity is necessary to promote the infiltration of cells, nerves and blood capillaries. In this work, a freeze drying method was used to prepare the porous scaffolds due to its simplicity and cost effectiveness.3,32 Further, the major advantage of this method is that it is a green technology wherein toxic solvents are not used. The interactions within gellan–HAP and amylopectin–HAP were expected to be similar to those found in the HAP and organic matrices present in natural bone.Thermogravimetric analysis
Thermogravimetric analysis profiles of the scaffold materials are shown in Fig. 1. The first weight loss observed between 30 °C and 130 °C for the scaffold material was due to the evaporation of residual water molecules. Further, the major weight loss between 200 °C and 489 °C for fMWCNT–gellan–HAP and 214 °C to 660 °C for fMWCNT–amylopectin–HAP was attributed to the thermal decomposition of gellan and amylopectin into carbonaceous material and pyrolysis of the labile oxygen-containing groups in the fMWCNTs. There was no significant weight loss above 700 °C. This indicated the thermal stability of HAP even above 700 °C.10,20,33,34 Further, the TG analysis indicated the presence of all the components in the prepared tricomponent scaffolds.FTIR analysis
FTIR analysis was carried out to identify the ionic interactions between the individual components in the tricomponent scaffolds and the results are shown in Fig. 2. All of the characteristic peaks for HAP at 3570, 1460, 1049, 966, 881 and 634 cm−1 were observed.12,20 Peaks at around 3138 cm−1, 1402 cm−1 and 1044 cm−1 were observed for the MWCNTs (Fig. S1†).34 The appearance of peaks corresponding to OH− stretching at 3500 cm−1, a carbon skeleton at 3138 cm−1, carbonyl (COOH) at 1638 cm−1, C–C stretching vibration at 1402 cm−1 and C–O stretching vibration at 1044 cm−1 confirmed the oxidation of the MWCNTs.3,22,35 The characteristic bands of OH− stretching at 3419 cm−1, C–H stretching at 2121 cm−1, asymmetric carboxylate at 1612 cm−1, symmetric carboxylate at 1400 cm−1 and pyranoside ring at 1047 cm−1 for gellan,31,36 and OH− stretching at 3445 cm−1, asymmetric C–H stretching at 2940 cm−1, adsorbed water at 1629 cm−1, angular deformation of C–H at 1399 cm−1, C–O ether stretching at 1090 cm−1 and C–O alcohol stretching at 1028 cm−1 for amylopectin were observed.20,37 In the FTIR spectra of the fMWCNT–gellan–HAP and fMWCNT–amylopectin–HAP scaffolds, all the characteristic peaks of gellan, amylopectin, HAP and the fMWCNTs were observed with a slight shift in peak values. The stretching vibrations of phosphate at 1049 cm−1 in pure HAP shifted to 1045 cm−1 and 1047 cm−1 in the fMWCNT–gellan–HAP and fMWCNT–amylopectin–HAP scaffold respectively, indicating strong hydrogen bonding between HAP and the fMWCNTs.41 Further, the synergistic effects of hydrogen bonding and ionic interaction between the polysaccharides (gellan and amylopectin), HAP and the oxygenated groups in the fMWCNTs were confirmed from their mixed characteristic peaks with reduced peak intensity. This also corroborated the high dispersion of HAP and the fMWCNTs in gellan and amylopectin and confirmed the formation of the composites.10,20,21,38XRD analysis
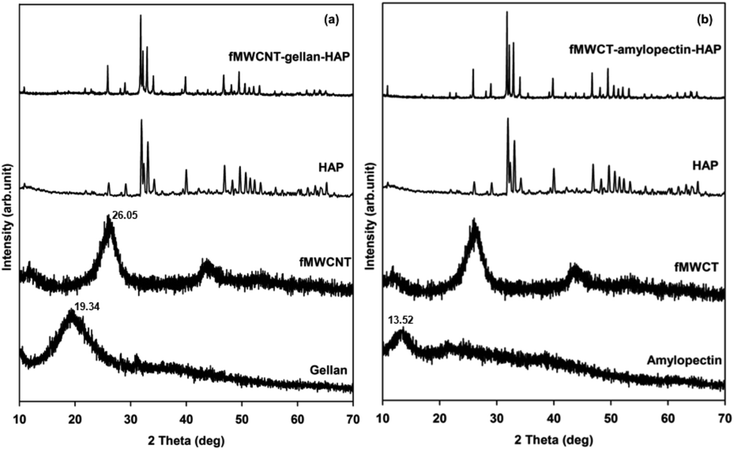
XRD analyses of gellan, amylopectin, fMWCNTs, HAP and the tricomponent scaffolds were carried out to identify the phase and crystallinity of the scaffolds and the results are shown in Fig. 3. The diffraction pattern of isolated HAP was found to be in good agreement with the standard value of HAP (Joint Committee on Powder Diffraction Standards (JCPDS)-09-0432/1996). The broad diffraction peaks obtained for the MWCNTs (25.71°) (Fig. S2†),34 fMWCNTs (26.05°), gellan (19.34°) and amylopectin (13.62°) indicated their amorphous nature with lower crystallinity.10,20 The diffraction pattern of the tricomponent scaffolds contains all the raw materials’ peaks with slight shifts in diffraction value and reduced intensity of the peaks for the fMWCNTs, gellan and amylopectin. The major peaks of HAP were shifted from 31.95° to 31.80°, 33.09° to 32.96°, 39.90° to 39.83°, and 49.65° to 49.47° in fMWCNT–gellan–HAP and from 31.95° to 31.79°, 33.09° to 32.93°, 39.90° to 39.82°, and 49.65° to 49.47° in fMWCNT–amylopectin–HAP, confirming the occurrence of interactions between the individual components in the scaffolds. The alignment of the gellan and amylopectin chains through intermolecular interactions was confirmed from the reduced intensity of their diffraction peaks.38 Further, the reduced intensity of the fMWCNTs’ peaks indicated the distribution of fMWCNTs in the tricomponent scaffolds.39 Furthermore, the amorphous nature observed in the diffraction of the scaffolds was due to the dispersion and interactions of crystalline HAP with amorphous fMWCNTs, gellan and amylopectin.20,21 These results confirmed the formation of tricomponent scaffolds with hydrogen and ionic bond interactions between the individual components as evidenced from FTIR.Morphological studies and porosity analysis
The porous nature and porosity of the scaffold are important parameters for tissue engineering applications. Higher porosity of the scaffold facilitates cell growth. FE-SEM images of HAP, and the fMWCNT–gellan–HAP and fMWCNT–amylopectin–HAP scaffolds are shown in Fig. 4. FE-SEM images of HAP (Fig. 4a and b) confirmed its crystalline nature with crystal sizes of 300–400 nm. Energy dispersive X-ray analysis of HAP (Fig. S1†) showed the presence of higher amounts of Ca and P with a small amount of carbon and is in good agreement with the reported values. The Ca/P ratio (2.05) of HAP was found to be slightly higher than that of its stoichiometric value.12 The morphology of fMWCNT–gellan–HAP (Fig. 4c and d) showed a lower porous nature with a pore size of 49–51 μm which might be attributed to the rigidity and higher viscous nature of the gellan. Whereas, the morphology of fMWCNT–amylopectin–HAP (Fig. 4e and f) showed a higher porous nature with a pore size 55–85 μm. Further, homogeneous dispersion of HAP on the scaffolds over the polysaccharide was clearly evident from the FE-SEM morphology (Fig. 4d and f). The porosity of the scaffolds was determined by the liquid displacement method and the value was found to be 82.82% in the case of fMWCNT–gellan–HAP and 91.76% for fMWCNT–amylopectin–HAP. The porosities of the prepared tricomponent scaffolds were high and they can hence serve as a good material for bone tissue engineering. Total porosity (such as ≥90%) is a crucial factor for the scaffolds. This provides sufficient opportunity for cell migration and expansion to maintain transport of nutrition and thereby influences in-growth of the bone tissue.38,40,41 In accordance the scaffold with the higher porosity (91.76%) fMWCNT–amylopectin–HAP facilitated higher cell proliferation.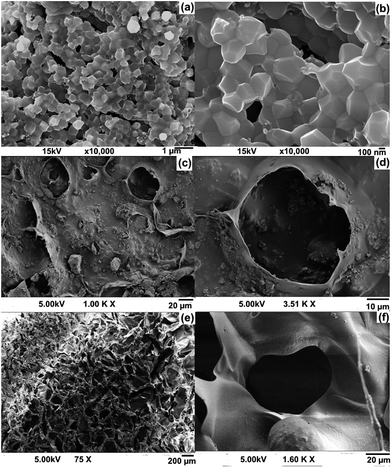 | ||
| Fig. 4 FE-SEM images of (a & b) HAP, and the (c & d) fMWCNT–gellan–HAP and (e & f) fMWCNT–amylopectin–HAP scaffolds. | ||
Compressive strength
One of the major hurdles in developing scaffolds for bone tissue engineering is the inverse correlation between material porosity and mechanical strength.42 An ideal scaffold for bone tissue engineering should provide good mechanical support within an appropriate physiological range until the broken bone is healed and it should also support the in-growth of bone tissue. In order to evaluate the mechanical strength of the scaffold, compressive strength tests were performed. Compressive strengths of the prepared sponge-like tricomponent scaffolds are shown in Fig. 5. The values were found to be 222.60 ± 8.4 kPa for fMWCNT–gellan–HAP and 57.32 ± 2.4 kPa for fMWCNT–amylopectin–HAP. The compressive strength value of the fMWCNT–gellan–HAP scaffold was found to be high due to the lower porosity and higher viscosity of gellan. The higher viscous nature of gellan enhanced the speed of the gelling of the reaction mixture and produced a rigid scaffold with lower porosity. The compressive strength values obtained for the tricomponent scaffolds are in agreement with those of previously reported scaffolds prepared by a freeze drying method.43–47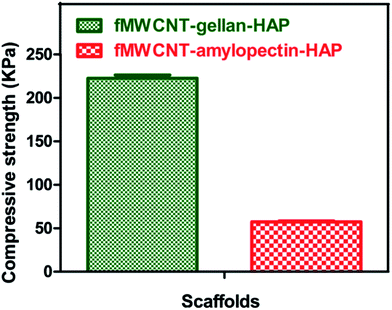 | ||
| Fig. 5 Compressive strength images of the tricomponent scaffolds. Values are expressed as mean ± standard deviation (n = 5). | ||
In vitro cell proliferation of MG 63 cells
An MTT assay for the prepared tricomponent scaffolds was performed to monitor cell proliferation and cytotoxicity. This assay was based on the ability of the mitochondria of live cells to reduce MTT into a purple formazan salt. Favorable cell attachment and spreading on the scaffold is an important requirement for better proliferation. Fig. 6 shows the cell proliferation results characterized by the MTT assay of fMWCNT–gellan–HAP and fMWCNT–amylopectin–HAP for 4 days. The MG 63 cells on the scaffolds were observed to have an increasing rate of cell proliferation over the culture time from day one to day four without any cytotoxicity. The increasing rate of cell proliferation was due to the increasing penetration of cells into the porous scaffold structure with increasing time. Further, this is in good agreement with previous results that indicated more cellular infiltration into highly porous 3D scaffolds. The higher porosity of the prepared tricomponent scaffolds can easily facilitate cell penetration and promote cell proliferation by providing more access to nutrients and oxygen for the innermost cells through the porous structure. Both scaffolds showed almost similar trends in their cell proliferation results because of the similar pore size exhibited by them. Moreover, previous studies including (MWCNT)–chitosan–HAP, MWCNT–gelatin–HAP, MWCNT–polypropylene–HAP and MWCNT–poly(L-lactic acid)–HAP scaffolds revealed that the CNTs promote osteoblast adhesion and proliferation. Cells cultured on CNT based scaffolds showed enhanced proliferation and adhesion.26–28,30 Hence, this evidences an important role of CNTs in the prepared tricomponent scaffolds for increased cell proliferation. These results suggested that the prepared tricomponent scaffolds are suitable to support the culture of MG 63 cells and confirmed the non-toxicity of functionalized CNTs. Hence, the prepared tricomponent scaffolds can facilitate cell proliferation and mimic the cells in native ECM for bone tissue engineering.48–50ALP activity
ALP is an enzyme used routinely as an early marker for differentiation of osteoblasts and hydrolyses phosphate to increase the local phosphate concentration and further plays a critical role to enhance the mineralization of natural ECM.51,52 ALP activity was measured by culturing MG 63 cells on the scaffolds after 2 and 4 days and is shown in Fig. 7. Similar to the results of the MTT assay, ALP activity also increased gradually with time. This indicated a commitment by the cells towards osteoblastic lineage. Biological processes of cells such as cell proliferation and differentiation are highly influenced by the interaction of physical surfaces with the cell boundaries.53 Further, previous studies have proved that MWCNTs could induce cell differentiation by absorbing more cell protein due to their larger surface area.54,55 ALP activity of cells cultured on the fMWCNT–gellan–HAP and fMWCNT–amylopectin–HAP scaffolds did not show any significant difference on 2nd and 4th days. The slightly higher ALP activity for the fMWCNT–amylopectin–HAP scaffold may be attributed to its higher porosity. This provided a positive indication of the mineralization of the ALP enzyme into an inorganic phosphate ion.48 | ||
| Fig. 7 ALP assay of the MG 63 cell line on the fMWCNT–gellan–HAP and fMWCNT–amylopectin–HAP scaffolds. Values are expressed as mean ± standard deviation (*P < 0.0001, n = 3). | ||
In vitro cell attachment assay
Hoechst 33342 DNA stain and Acridine Orange dye can show the live cells under a fluorescence microscope and have been used to study the cell attachment of MG 63 on the tricomponent scaffolds. The interactions and adhesion of cells on the scaffold influence the rate and quality of new tissue formation.38 From the fluorescence microscopy images using Hoechst stain (Fig. 8a and b) and Acridine Orange (Fig. 8c and d), it can be observed that osetoblasts like MG 63 cells are well adhered and proliferated on the scaffolds and remained viable after 4 days. The cells on the fMWCNT–amylopectin–HAP scaffold (Fig. 8b and d) appeared to be denser compared with those on the fMWCNT–gellan–HAP scaffold (Fig. 8a and c) due to the higher porosity of fMWCNT–amylopectin–HAP. Further, the hydrophilic groups on the fMWCNTs and polysaccharides have the ability to interact with cell proteins and provide sites to promote cell adhesion by retaining and recruiting cells onto the scaffold surface. These results suggest that the scaffold materials possess an adequate pore size and porosity that allows the cells to grow by sufficient diffusion of nutrients and oxygen.56,57Experimental
Materials
HAP was isolated from chicken bones (purchased from a local slaughterhouse) by a thermal calcination method. MWCNTs (outer diameter < 8 nm, length 10–30 μm) were purchased from http://cheaptubes.com, USA. The MG 63 cell line was obtained from the National Center for Cell Science, Pune, India. Gellan gum, amylopectin, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), and the Acridine Orange and bis-benzimide Hoechst 33342 stains were purchased from Sigma Aldrich. Dulbecco’s Modified Eagle’s Medium (DMEM) was purchased from HIMEDIA. Sodium phosphate buffer, NaCl and KCl were purchased from Merck. NaOH, conc. HNO3 and conc. H2SO4 (AR grade) were purchased from S. D. Fine Chemicals.Isolation of HAP from chicken bone
HAP was isolated by thermal calcination as reported previously by our research group.12 The chicken bones were washed with concentrated NaOH solution followed by rinsing with water to remove traces of meat, skin and other impurities present on the surface of the bones. The bones were then dried in a hot air oven at 100 °C and ground into small pieces. Pretreated chicken bones were subjected to thermal calcination at 800 °C with a 20 h holding time in an electric muffle furnace (SUNSIM, India).Oxidation of MWCNTs
MWCNTs were oxidized by a modified procedure reported by Ann et al.58 The MWCNTs were sonicated in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of conc. HNO3 and conc. H2SO4 for 20 min. Then the mixture was refluxed for 3 h. The fMWCNTs were centrifuged, washed with distilled water and dried in oven at 100 °C overnight.
1 mixture of conc. HNO3 and conc. H2SO4 for 20 min. Then the mixture was refluxed for 3 h. The fMWCNTs were centrifuged, washed with distilled water and dried in oven at 100 °C overnight.
Preparation of the fMWCNT–polysaccharide–HAP scaffolds
1.74 g of polysaccharide (gellan gum or amylopectin) was dissolved in 100 mL of double distilled water. 60 mg of the fMWCNTs was dispersed in distilled water by sonication and added dropwise to the polysaccharide solution which was then stirred for 3 h. 4.2 g of isolated HAP suspended in 50 mL of distilled water was then added slowly to the fMWCNT–polysaccharide solution and the resultant solution was stirred for 24 h. The resultant composite solution mixture was transferred into 12 well plates with 2 g solution per well. The samples were freeze dried at −80 °C and lyophilized with a freeze dryer to form the scaffolds.General characterization
The thermal stability of the scaffolds was studied using TG analysis (SDTQ 600 TA Instrument, USA) with a scan range of 50 °C to 900 °C at a constant heating rate of 10 °C min−1 under a nitrogen atmosphere. The characteristic stretching and bending frequencies of the samples were measured in a FTIR spectrometer (Jasco FTIR4100, Japan) by recording the spectrum over the range of 4000 cm−1 to 500 cm−1 using KBr. The phase and the crystallinity of the samples were examined by XRD (Bruker, D8 Advance X-ray diffraction spectrophotometer, Germany) at room temperature using CuKα as the radiation source with a wavelength of 1.504 Å, over an angle range of 10° to 80°, a step size of 0.02° and a scan speed of 0.5° min−1. The resultant XRD profile of the isolated HAP was compared with the JCPDS cards available in the system’s software. Further, the morphology of the tricomponent scaffolds was studied by FE-SEM (SUPRA 55, Carl Zeiss, Germany) analysis.Porosity measurements
Porosity is an important property for cell proliferation. The total porosity of the scaffold was determined using the liquid displacement method.20 Initially, the volume of ethanol and weight of the dry scaffold were measured. Then, the scaffold was immersed in dry ethanol for 48 h. After 48 h, the scaffold was taken out from the ethanol and the weight of the scaffold was measured. The total porosity was calculated using the equation| Porosity = (W1 − W3)/(W2 − W3) |
Compressive strength
The compressive strength of the freeze dried scaffolds was obtained using a universal testing machine (UTM). The test was performed using an H5KS system (Tinius Olsen, Salfords, UK). A 5000 N load cell with the standard grips of crosshead speed of 0.5 mm min−1 was used for the compression measurements. A determination was made using five replicates.In vitro cell proliferation assay of the scaffolds
The MG-63 cells were plated separately in 96 well plates at a concentration of 1 × 105 cells per well. After 24 h, the cells were washed twice with 100 μL of serum-free medium and starved for an hour at 37 °C. After starvation, the cells were added onto the scaffold material which had previously been sterilized with 75% alcohol followed by 100% alcohol. Cells treated without scaffold material were used as a control. At the end of the treatment period of 1, 2, 3 and 4 days, the medium was aspirated and a serum free medium containing MTT (0.5 mg mL−1) was added and incubated for 4 h at 37 °C in a CO2 incubator. The determinations were performed using three replicates each time.The MTT containing medium was then discarded and the cells were washed with phosphate buffer solution (PBS) (200 μL). The formed formazan crystals were then dissolved by adding 100 μL of DMSO and this was mixed thoroughly by pipetting up and down. Absorbance of the purple blue formazan dye was measured in a microplate reader at 570 nm (Biorad 680).20 Cytotoxicity was determined using GraphPad Prism 5 software.
Alkaline phosphatase assay
To determine ALP activity, cells were cultured as per the cytotoxicity analysis. After incubation with the scaffolds for 2 and 4 days, the cells were treated with 10 μL (100 mmol L−1) of p-nitrophenyl phosphate and incubated for 30 min at 37 °C in a CO2 incubator. ALP expressed by the cells can hydrolyze the p-nitrophenyl phosphate into p-nitrophenol and inorganic phosphate. The released p-nitrophenol absorbance was measured at 405 nm spectrophotometrically.59 The determination was made using three replicates each time.Hoechst stain assay for cell attachment
Hosechst 33342 DNA staining has been used to identify cell attachment and growth on scaffold material.20 For this, the ethanol sterilized scaffold material was placed in a cell culture plate, seeded with MG 63 cells and incubated. After 4 days of culturing, the medium was removed from the wells and the cells were washed with PBS solution. The cells were stained with 0.5 mL of Hoechst 33342 solution (3.5 μg mL−1 in PBS) and incubated for 30 min at 37 °C. After 30 min, the Hoechst stained cells were visualized and photographed under a microscope (Olympus-version-6.0, Carl Zeiss lens, Germany).Acridine Orange stain assay for cell attachment
Acridine Orange is used to identify both viable and apoptotic cells on scaffold materials.60 For this, the ethanol sterilized scaffold material was placed in a cell culture plate, seeded with MG 63 cells and incubated. After 4 days of culturing, the medium was removed from the wells and the cells were washed with PBS solution. The cells were stained with 200 μL of dye mixture (100 μL mg−1 Acridine Orange distilled water). The suspension was immediately examined and viewed under a microscope (Olympus-version-6.0, Carl Zeiss lens, Germany).Statistical analysis
Statistical analysis for the MTT and ALP assays and compressive strength were performed using GraphPad Prism 6 software. Results are presented as mean ± standard deviation.Conclusions
In this study new tricomponent scaffolds were developed using fMWCNTs, natural polysaccharides and biocompatible HAP. For the first time, fMWCNT–gellan–HAP and fMWCNT–amylopectin–HAP scaffolds were prepared by a freeze drying method and characterized. Natural polysaccharides, gellan gum and amylopectin play a major role in mimicking the ECM. The essential criterion of porous morphology for bone tissue engineering was confirmed from FE-SEM images of the scaffolds. In vitro MTT assays for cell proliferation and Hoechst 33342 DNA and Acridine Orange stains for cell adhesion studies of the MG 63 cell line confirmed that the prepared scaffolds attempted to mimic the ECM and provide a temporary template for the growth of target tissues. ALP activity of the scaffolds confirmed better mineralization of the scaffolds. The compressive strength values of the scaffolds were in agreement with those for previously reported scaffolds prepared by a freeze drying method. The high degree of interaction between the organic and inorganic matrices was confirmed by FTIR and XRD analysis. The chemical composition of the prepared scaffolds was similar to that of bone. Even though there are reports on the toxicity of CNTs, there are also reports of improved cell viability with functionalized CNTs. The functionalized CNTs provide selective absorption and attachment of proteins from cell culture media. Oxidation of CNTs introduces carboxyl and hydroxyl groups which further enhance the stronger interactions with proteins through hydrogen bonding. This has justified the inclusion of CNTs in our scaffolds and has shown good cell proliferation and positive indication for mineralization of the MG 63 cells on the scaffolds. Moreover, CNTs have improved the compressive strength of the prepared tricomponent scaffolds. In conclusion, the prepared tricomponent scaffolds can serve as better biomaterials for bone tissue engineering.Acknowledgements
The authors gratefully acknowledge the management of the Karpagam College of Engineering, Coimbatore for their encouragement and support. The authors also acknowledge the powder XRD facility at SAS, VIT University, jointly funded by VIT and FIIST, DST India.References
- K. J. L. Burg, S. Porter and J. F. Kellam, Biomaterials, 2001, 12, 2347–2359 Search PubMed.
- T. Long, Y.-T. Liu, S. Tang, J.-L. Sun, Y.-P. Guo and Z.-A. Zhu, J. Biomed. Mater. Res., Part B, 2014, 102, 1740–1748 CrossRef PubMed.
- J. Venkatesan, Z.-J. Qian, B. M. Ryu, N. A. Kumar and S. K. Kim, Carbohydr. Polym., 2011, 83, 569–577 CrossRef CAS.
- E. Murugan and S. Arumugam, RSC Adv., 2014, 4, 35428–35441 RSC.
- R. Rajesh and Y. D. Ravichandran, Int. J. Nanomed., 2015, 10, 7–15 CAS.
- A. J. Salgado, O. P. Coutinho and R. L. Reis, Macromol. Biosci., 2004, 4, 743–765 CrossRef CAS PubMed.
- A. R. Shrivats, M. C. McDermott and J. O. Hollinger, Drug Discovery Today, 2014, 19, 781–786 CrossRef CAS PubMed.
- X. Liu and P. X. Ma, Ann. Biomed. Eng., 2004, 32, 477–486 CrossRef PubMed.
- K. Rezwan, Q. Z. Chen, J. J. Blaker and A. R. Boccaccini, Biomaterials, 2006, 27, 3413–3431 CrossRef CAS PubMed.
- R. Rajesh and Y. D. Ravichandran, RSC Adv., 2015, 5, 41135–41143 RSC.
- A. Oyefusi, O. Olanipekun, G. M. Neelgund, D. Peterson, J. M. Stone, E. Williams, L. Carson, G. Regisford and A. Oki, Spectrochim. Acta, Part A, 2014, 132, 410–416 CrossRef CAS PubMed.
- R. Rajesh, A. Hariharasubramanian and Y. D. Ravichandran, Phosphorus, Sulfur Silicon Relat. Elem., 2012, 187, 914–925 CrossRef CAS.
- H. Shin, S. Jo and A. G. Mikos, Biomaterials, 2003, 24, 4353–4364 CrossRef CAS PubMed.
- S.-J. Seo, J.-J. Kim, J.-H. Kim, J.-Y. Lee, U. S. Shin, E.-J. Lee and H.-W. Kim, Compos. Sci. Technol., 2014, 96, 31–37 CrossRef CAS.
- F. Gholami, S. H. S. Zein, S. B. Ismail and S. H. Tan, Chem. Eng. Sci., 2014, 2, 1–14 CrossRef.
- S. Saravanan, S. Nethala, S. Pattnaik, A. Tripathi, A. Moorthi and N. Selvamurugan, Int. J. Biol. Macromol., 2011, 49, 189–193 CrossRef PubMed.
- J. T. Oliveira, l. Martins, R. Picciochi, P. B. Malafaya, R. A. Sousa, N. M. Neves, J. F. Mano and R. L. Reis, J. Biomed. Mater. Res., Part A, 2010, 93, 852–863 CAS.
- L. P. da Silva, M. T. Cerqueria, R. A. Sousa, R. L. Reis, V. M. Correlo and A. P. Marques, Acta Biomater., 2014, 10, 4787–4797 CrossRef CAS PubMed.
- L. Stobinski, P. Tomasik, C.-Y. Lii, H.-H. Chan, H.-M. Lin, C.-T. Kao and K.-S. Lu, Carbohydr. Polym., 2003, 51, 311–316 CrossRef CAS.
- J. Venkatesan, R. Pallela, I. Bhatnagar and S.-K. Kim, Int. J. Biol. Macromol., 2012, 51, 1033–1042 CrossRef CAS PubMed.
- S. Pok, F. Vitale, S. L. Eichmann, O. M. Benavides, M. Pasquali and J. G. Jacot, ACS Nano, 2014, 8, 9822–9832 CrossRef CAS PubMed.
- R. Rajesh, A. Hariharasubramanian, N. Senthilkumar and Y. D. Ravichandran, Int. J. Pharm. Pharm. Sci., 2012, 4, 716–720 CAS.
- A. Hariharasubramanian, Y. D. Ravichnadran, R. Rajesh, K. R. Kumar, M. Kanagaraj and S. Arumugam, Mater. Chem. Phys., 2014, 143, 838–844 CrossRef CAS.
- K. Sahithi, M. Swetha, K. Ramasamy, N. Srinivasan and N. Selvamurugan, Int. J. Biol. Macromol., 2010, 46, 281–283 CrossRef CAS PubMed.
- A. Barrientos-Duran, E. M. Carpenter, N. I. zur Nieden, T. I. Malinin, J. C. Rodriguez-Manzaneque and L. P. Zanello, Int. J. Nano Dimens., 2014, 9, 4277–4291 CrossRef PubMed.
- L. Chen, J. Hu, X. Shen and H. Tong, J. Mater. Sci.: Mater. Med., 2013, 24, 1843–1851 CrossRef CAS PubMed.
- I.-K. Yoon, J.-Y. Hwang, J.-W. Seo, W.-C. Jang, H.-W. Kim and U. S. Shin, Carbon, 2014, 77, 379–389 CrossRef CAS.
- C. Z. Lio, K. Li, H. M. Wong, W. Y. Tong, K. W. K. Yeung and S. C. Tjong, Mater. Sci. Eng., C, 2013, 33, 1380–1388 CrossRef PubMed.
- B. Dorj, J.-E. Won, J.-H. Kim, S.-J. Choi, U. S. Shin and H.-W. Kim, J. Biomed. Mater. Res., Part A, 2013, 101, 1670–1681 CrossRef PubMed.
- F. Mei, J. Zhong, X. Yang, X. Ouyang, S. Zhang, X. Hu, Q. Ma, J. Lu, S. Ryu and X. Deng, Biomacromolecules, 2007, 8, 3729–3735 CrossRef CAS PubMed.
- J. Roman, M. V. Cabanas, J. Pena and M. V. Regi, Sci. Technol. Adv. Mater., 2011, 12, 045003 CrossRef.
- N. Barbani, G. D. Gurrea, C. Cristallini, P. Urciuoli, R. Avvisati, A. Sala and E. Rosellini, J. Mater. Sci.: Mater. Med., 2012, 23, 51–61 CrossRef CAS PubMed.
- N. Barbani, M. L. Coluccio, C. Cristallini, G. D. Guerra and E. Rosellini, J. Appl. Polym. Sci., 2010, 118, 3131–3140 CrossRef CAS.
- R. Rajesh, Y. D. Ravichandran, N. A. N. Raj and N. Senthilkumar, Polym.-Plast. Technol. Eng., 2014, 53, 1105–1110 CrossRef CAS.
- A. Hariharasubramnian, Y. D. Ravichandran, R. Rajesh, R. Rajakumari, G. K. Selvan and S. Arumugam, Fullerenes, Nanotubes, Carbon Nanostruct., 2014, 22, 874–886 CrossRef.
- G. Ciardelli, V. Chiono, G. Vozzi, M. Pracella, A. Ahluwalia, N. Barbani, C. Cristallini and P. Giusti, Biomacromolecules, 2005, 6, 1961–1976 CrossRef CAS PubMed.
- D. C. Dragunski and A. Pawlicka, Mater. Res. Bull., 2001, 4, 77–81 CrossRef CAS.
- M. Li, Y. Wang, Q. Liu, Q. Li, Y. Cheng, Y. Zheng, T. Xi and S. Wei, J. Mater. Chem. B, 2013, 1, 475–484 RSC.
- G. Yang, J. Su, J. Gao, X. Hu, C. Geng and Q. Fu, J. Supercrit. Fluids, 2013, 73, 1–9 CrossRef CAS.
- S. M. Bose, M. Roy and A. Bandyopadhyay, Trends Biotechnol., 2012, 30, 546–554 CrossRef CAS PubMed.
- S. Yunoki, T. Ikoma, A. Monkawa, E. Marukawa, S. Sotome, K. Shinomiya and J. Tanaka, J. Biomater. Sci., Polym. Ed., 2007, 18, 393–409 CrossRef CAS PubMed.
- H. R. R. Ramay and M. Zhang, Biomaterials, 2004, 25, 5171–5180 CrossRef CAS PubMed.
- M. Kawaguchi, T. Fukushima, T. Hayakawa, N. Nakashima, Y. Inoue, S. Takeda, K. Okamura and K. Taniguchi, Dent. Mater. J., 2006, 25, 719–725 CrossRef CAS.
- X. Shi, B. Sitharaman, Q. P. Pham, F. Liang, K. Wu, W. E. Billups, L. J. Wilson and A. G. Mikos, Biomaterials, 2007, 28, 4078–4090 CrossRef CAS PubMed.
- G. Jell, R. Verdejo, L. Safinia, M. S. P. Shaffer, M. M. Stevens and A. Bismarck, J. Mater. Chem., 2008, 18, 1865–1872 RSC.
- X. Wu, Y. Liu, X. Li, P. Wen, Y. Zhang, Y. Long, X. Wang, Y. Guo, F. Xing and J. Gao, Acta Biomater., 2010, 6, 1167–1177 CrossRef CAS PubMed.
- O. Lm, J. Li, M. Wang, L. G. Zhang and M. Keidar, Int. J. Nanomed., 2012, 7, 2087–2099 Search PubMed.
- H.-J. Lee, S.-H. Ahn and G. H. Kim, Chem. Mater., 2012, 24, 881–891 CrossRef CAS.
- S. Cai, H. Su, Q. Jiang and Y. Yang, Langmuir, 2013, 29, 2311–2318 CrossRef CAS PubMed.
- A. Abbarrategi, M. C. Gutierrez, C. Moreno-Vicente, M. J. Hortiguela, V. Ramos, J. L. Lopez-Lacomba, M. L. Ferrer and F. del Monte, Biomaterials, 2008, 29, 94–102 CrossRef PubMed.
- B. A. Allo, S. Lin, K. Mequanint and A. S. Rizkalla, ACS Appl. Mater. Interfaces, 2013, 5, 7574–7583 CAS.
- I. Rajzer, E. Menaszek, R. Kwiatkowski, J. A. Planell and O. Castano, Mater. Sci. Eng., C, 2014, 44, 183–190 CrossRef CAS PubMed.
- L. Pan, X. Pei, R. He, Q. Wan and J. Wang, Colloids Surf., B, 2012, 93, 226–234 CrossRef CAS PubMed.
- C. Li, Y. Wang, Y. Lai, W. Yang, F. Jiao, H. Zhang, S. Ye and Q. Zhang, Colloids Surf., B, 2011, 83, 367–375 CrossRef PubMed.
- X. Li, H. Liu, X. Niu, B. Yu, Y. Fan, Q. Feng, F.-Z. Cui and F. Watari, Biomaterials, 2012, 33, 4818–4827 CrossRef CAS PubMed.
- D. Milovac, T. C. Gamboa-Martinez, M. Ivankovic, G. G. Ferrer and H. Ivankovic, Mater. Sci. Eng., C, 2014, 42, 264–272 CrossRef CAS PubMed.
- D. Depan, B. Girase, J. S. Shah and R. D. K. Misra, Acta Biomater., 2011, 7, 3432–3445 CrossRef CAS PubMed.
- J. S. Ann, B. U. Nam, S. H. Tan and S. C. Hang, Macromol. Symp., 2007, 249–250, 267–282 Search PubMed.
- J. Venkatesan and S.-K. Kim, J. Biomed. Nanotechnol., 2012, 8, 676–685 CrossRef CAS PubMed.
- D. Baskic, S. Popovic, P. Ristic and N. N. Arsenijevic, Cell Biol. Int., 2006, 30, 924–932 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra16709h |
| This journal is © The Royal Society of Chemistry 2016 |