Rutile nanotubes by electrochemical anodization†
Abstract
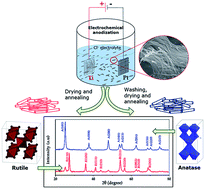
We present a facile method to synthesize rutile titanium dioxide nanotubes (R-TiNT), directly in powder form through rapid breakdown electrochemical anodization by modifying the post anodization processing and annealing temperature. Photocatalytic activity of the R-TiNT was examined by evaluating the degradation of rhodamine B dye in visible light.

 Please wait while we load your content...
Please wait while we load your content...