A comprehensive study of methane/carbon dioxide adsorptive selectivity in different bundle nanotubes†
Hamed Akbarzadeh* and
Mohsen Abbaspour
Department of Chemistry, Hakim Sabzevari University, Sabzevar, Iran. E-mail: akbarzadehhamed@yahoo.com
First published on 18th July 2016
Abstract
We have investigated methane/carbon dioxide adsorptive selectivity in carbon nanotube (CNT) and silicon carbide nanotube (SiCNT) bundles via MD simulations. Many different effects have been examined and discussed in this research such as the pressure effect, the effect of the kind of nanotube bundle, the nanotube bundle diameter and chirality effects.
1. Introduction
Emission of greenhouse gases, particularly CO2, has been recognized by the overwhelming majority of the scientific community as one of the most important factors affecting global warming.1 Carbon dioxide is primarily emitted by fossil fuel combustion, natural gas sweetening, syngas production and some chemical plants.2 According to a report by the Intergovernmental Panel on Climate Change (IPCC), the concentration of CO2 in the atmosphere had been increased from preindustrial times to 1999 by about 31%.3 Due to these reasons, development of processes for the separation and capture of CO2 from the atmosphere, fuel gas, landfill and natural gas and other sources remains a topic of considerable interest due to the prominent role of CO2 in global climate change.4Several methods have been proposed to separate carbon dioxide from gas mixtures, including amine and ionic liquid absorption,5–7 chemical conversion,5 membrane separation,8 and adsorption by nanoporous materials.9 Physisorption-based separation methods, using porous materials as molecular sieves, are among the most environmental friendly and economically feasible methods to achieve efficient removal of carbon dioxide from CO2 + CH4 mixtures.10 The most common types of porous materials which have been used as adsorbents are carbon-based molecular sieves,11–16 zeolites,17–20 polymeric membranes,21–23 metal–organic frameworks,24–29 covalent organic frameworks,30,31 and silicalite.32 For example, Yi et al.15 obtained the adsorption equilibrium isotherms of carbon dioxide, methane and nitrogen on microwave activated carbon (MAC) at three temperatures (298, 308 and 323 K) by a static volume instrument. Their results indicated that CO2 still dominate the adsorption system in the ternary mixtures. Billemont et al.16 studied the adsorption of carbon dioxide, methane, and their mixtures in nanoporous carbons in the presence of water using experiments and molecular simulations. Basu et al.27 used both dense and asymmetric metal organic frameworks (MOFs) containing membranes for CO2/CH4 and CO2/N2 selectivity. Mishra et al.28 reported the gas adsorption properties of a zinc based MOFs for four industrially relevant gases (CO2, CO, CH4 and N2) up to 26 bar at three different temperatures (294, 314 and 350 K) using a standard gravimetric method. Zhang et al.29 performed a computational study to evaluate the adsorption-based separation of CO2 from flue gas (mixtures of CO2 and N2) and natural gas (mixtures of CO2 and CH4) using microporous MOF. Their results showed that electrostatic interactions can greatly enhance the separation efficiency of this MOF for gas mixtures of different components.
Carbon-based materials (such as carbon nanotubes (CNTs) and silicon carbide nanotubes (SiCNTs)) possess a superior structural stability to a wide range of processing conditions, keeping them in the race for commercial applications.10 The SiCNTs possess interesting properties and, naturally, draw comparison with their carbonaceous counterparts, namely, the carbon nanotubes (CNTs).33 Some experimental and theoretical studies have been carried in order to investigate gas storage capacity of the SiCNTs. The tubule formation of the SiCNTs and the existence of specific charge arrangement inside their surface make them attractive alternative materials for gas storage (relative to the CNTs).33 Recently, Malek and Sahimi33 employed molecular dynamics (MD) simulations in order to examine the adsorption and diffusion of different gases including CO2 and CH4 in the SiCNTs, as a function of the pressure and the type of the nanotubes, namely, the zigzag, armchair, and chiral tubes. Their simulations indicated the nanotubes' chirality and curvature have strong effects of on the pressure dependence of the adsorption isotherms and the self-diffusivities. They investigated the gas adsorptions alone and they did not investigate the separation of the CO2/CH4 mixture in their work. More recently, Baghernia and Shadman34 reported CH4 and CO2 adsorption in SiCNTs using grand canonical Monte-Carlo (GCMC) and calculated the heat of gas adsorption. Their results demonstrated that at ambient temperature and high pressure, the gas adsorption is in the order of CO2 > CH4 and the nanotubes' order is (10,10) < (20,20) < (40,40). But, they did not investigate the separation of the CO2/CH4 mixture in their work. They did not also tested the CNT or SiCNT bundles for the gas adsorptions. They did not also investigated the nanotube chirality effect in the separation of the gases.
For the first time, we have performed molecular dynamics (MD) simulations to investigate the separation and diffusion of a CO2 + CH4 equimolar mixture using silicon carbide nanotube (SiCNT) bundles as a potential molecular sieve. This research is of critical importance in natural gas recovery and refinement.35 We have also studied the effects of the size and chirality of the SiCNT bundles and the temperature and pressure effects on the results. Such a fundamental investigation has been not performed before. Another major goal of the present study is to compare the performance of the SiCNT bundles with that of the CNT bundles.
2. Simulation details
The main aim of the present study was to investigate the separation of an equimolar CO2/CH4 mixture at different temperatures (280, 300, 320 and 350 K) and pressures (1, 3, 5, 7, 9, 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 atm) in the SiCNTs by employing MD simulations. For a certain pressure value, the same number of CO2 and CH4 molecules were placed into the simulation box. Because the ensemble was NVT, we calculated the density of gas mixture by knowing the number of the molecules and the volume of the cell. Then, the pressure of the mixture was obtained using the equation of state36 by knowing the density and temperature. We have also changed the pressure of the mixture by changing the number of the gas molecules.In order to perform this simulation study, the initial configuration of the system was built by initially placing the cubic SiCNTs in the center of the simulation box with hexagonal arrangement (Fig. 1a).1,10,37–39 From both sides of the SiCNT (along the z-axis direction) two orthogonal parallelepiped boxes were placed, having the same x- and y-axis length with the cubic SiCNT (approximately 50 Å) and the double z-axis length.1,10 Each of the boxes was containing a well equilibrated configuration of a mixture with equal numbers of CO2 and CH4 molecules at the desired temperature and pressure (Fig. 1b).1,10 These equilibrated configurations were obtained by performing a NVT-MD simulation of the bulk mixture for a time period of 1 ns.
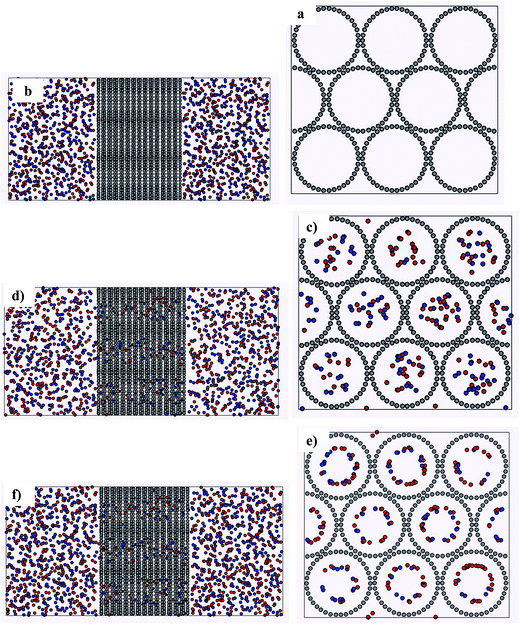 | ||
| Fig. 1 The snapshots of the simulation steps of the separation of CO2 + CH4 mixture in silicon-carbide nanotube bundles at 300 K. | ||
After calculating the amount of adsorbed molecules in the bundle nanotube, several subsequent 4 ns MD run were performed. In these runs, the bundle nanotube with the adsorbed gas molecules were placed at the center of the simulation box. Then, the bulk gas mixture (which were initially used in the first configuration) was again placed at the left and right of the system (Fig. 1c and d) and the whole of the system was equilibrated.1,10 The equilibration is achieved when the total energy of the system and also, the number of the adsorbed molecules in the nanotube bundle are constant with the time.
From each simulation, the amount of adsorbed molecules was calculated and this procedure was repeated several times until the amount of adsorbed CO2 and CH4 reached a constant value and the saturation was achieved (Fig. 1e and f).1,10 This procedure of consecutive MD simulations until the SiCNT is saturated can be seen as a stationary flux of gas flowing over a SiCNT.1,10 In this sense the results obtained by this method can be considered as equivalent with the ones obtained with the commonly employed Grand Canonical Monte Carlo (GCMC), since in this method the main idea is to bring the material in equilibrium with a virtual gas reservoir and to insert or delete gas molecules until the value of the chemical potential inside the porous material becomes equal to that one of the bulk gas.1,10 In such a way this stationary flux is being achieved.1,10,38,39
To keep temperature constant, the Nose–Hoover thermostat40 with a relaxation time of 0.1 ps was applied. The equations of motion were integrated using the Verlet leapfrog algorithm with a time step of 1 fs. The analysis of simulated trajectories and calculation of different properties were performed using the utilities of DL_POLY 4.03 program.41
Models for CH4 and CO2 were taken from the TraPPE force field of Siepmann and co-workers.42,43 Both models have been optimized to accurately reproduce the experimental vapor–liquid equilibrium envelopes of the pure substances. The interactions parameters between atoms in the SiCNT, bonded part and non-bonded part (coulombic and van der Waals interactions, partial charges and parameters for the repulsive and attractive parts of the interactions), were applied using the GROMOS 96 force field.33,44,45 The values of the parameters for the different interactions have been presented in Table 1. Interaction parameters between unlike Lennard-Jones sites were also estimated using the Lorentz–Berthelot combining rules. It should be also noted that the electronic structure of very large nanotubes, for instance (40,40), will be different from tighter ones (10,10) and so, they have the different potential parameters.46 The nanotubes with the different chiralities also have the different potential parameters but for simplicity and such as the previous works,33 we have considered the same potential parameters for the different nanotubes in this work.
| Atom (molecule) | σ (Å) | ε/k (K) |
|---|---|---|
| C(CNT) | 3.55 | 28.1 |
| C(CO2) | 2.76 | 28.1 |
| O(CO2) | 3.03 | 80.5 |
| Si | 3.39 | 294.5 |
| CH4 | 3.73 | 148.0 |
3. Results and discussion
3.1 Separation isotherms
In order to present a quantitative measurement of the separation of the CO2 + CH4 mixture, the adsorption selectivity has been calculated by using the following equation:4,10,47
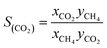 | (1) |
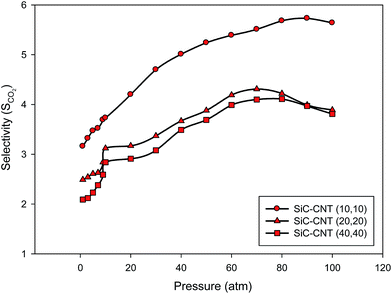 | ||
| Fig. 2 The CO2 selectivity as a function of the total pressure in the different armchair SiCNT bundles at 300 K. | ||
According to this figure, the SCO2 is always greater than unity since CO2 is preferentially adsorbed over CH4 which is due to its stronger interaction with the pore walls. These interactions are of the van der Waals interactions which they have been performed using the LJ potential in the simulations. For careful observation, the actual values have been presented in Table S1 in the ESI.† The similar observations have been reported in the CO2 + CH4 mixture using different carbon-based nanoporous by Palmer et al.4 and Skarmoutsos et al.10 The maximum value of the adsorption selectivity is 5.73 (Fig. 2), signifying that the composition of the CO2 + CH4 mixture inside the SiCNT bundles is about 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Such a finding indicates that the SiCNT bundles could be used as a more suitable candidate material to tune the composition of CO2 + CH4 mixtures via adsorption than other carbon-based nanoporous structures.10
1. Such a finding indicates that the SiCNT bundles could be used as a more suitable candidate material to tune the composition of CO2 + CH4 mixtures via adsorption than other carbon-based nanoporous structures.10
In the zero to moderate-pressure regime (0–20 atm), the selectivity of carbon dioxide passes through an inflection. In the other words, the selectivity increases sharply at low pressures and then steadily increases at moderate pressures until a maximum is reached and then decreases a little. The sudden increase of the selectivity at low pressures is caused by a sharp increase in the amount of CO2 adsorption. As CO2 adsorption begins to increase, the amount of CH4 adsorption decreases as a result of the competition between the two species for the unoccupied void space. This is expected since it has been shown that in the low pressure limit, the selectivity are only dependent on the adsorbate–adsorbent interactions. But, at moderate and high pressures, the distance between the molecules are in the range of the repulsion forces more than the attractive forces and so, the adsorption value (and the selectivity) is not as much as the value at lower pressures. In the other words, the adsorbate–adsorbent interactions are not very important at moderate and high pressures as they are significant in the low pressure limit. The same conclusions have been also obtained by Palmer et al.4 for the adsorptive behavior of CO2 + CH4 mixture in a carbon nanospace using molecular simulations.
According to Fig. 2, in the larger nanotube bundles, the SCO2 is relatively low which may be due to the weaker adsorbate–adsorbent interactions. The SCO2 increases with increasing the pressure until a maximum is reached and then decreases a little. This maximum also occurs in all of the different nanotubes. This suggests that SCO2 decreases as a result of an increase in density inside the pores, which entropically favors CH4 over CO2 because of its spherical shape and efficient packing geometry. As the pore width decreases, the external field contributions due to each of the opposing pore walls begin to overlap and a sharp increase in the SCO2 occurs.4
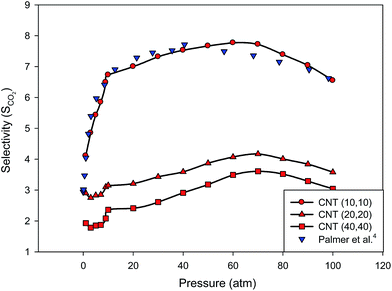
In order to compare our results in the SiCNT bundles with the CNT bundles, we have also presented the CO2 selectivity in the different armchair CNT bundles at 300 K in Fig. 3. According to Fig. 2 and 3, the smaller CNTs adsorb more CO2 than the SiCNT, but the larger CNTs adsorb less carbon dioxide than the SiCNTs, particularly at higher pressures. These results are in good agreement with those of Malek and Sahimi33 in the CNTs and SiCNTs. The difference in the gas storage capacities of the CNTs and SiCNTs may be due to the different orientation of the gas molecules toward various binding site, such as the atoms, as well as the bonds and the centers of the hexagons.33,48 We have also compared our results of SCO2 in the (10,10) CNT bundles with those results of Palmer et al.4 obtained using the GCMC simulations in Fig. 3. As this figure shows, there is good agreement between our MD results and the GCMC results of Palmer et al.4 in the (10,10) CNT bundle which confirms our method of simulations.
We have also compared the CO2 selectivity in the armchair and zigzag SiCNT and CNT bundles at 300 K in Fig. 4. According to this figure, the selectivity isotherm in the zigzag nanotube exhibits sharp increase in the adsorption which may be due to the stronger interactions between the CO2 molecules and the zigzag walls than the armchair wall. Thus, the chirality of the CNTs and SiCNTs has a significant effect on their sorption capacity for CO2. Please note that in the applied force field, the nanotube–gas pair potential is the same for both the armchair and zigzag nanotubes, however, a different arrangement of the nanotube atoms relative to the gas molecules in armchair and zigzag tubes leads to different gas–tube interactions. Malek and Sahimi33 also reported that the distinct structures of the two types of the nanotubes greatly influence the behavior of gases in such nanostructured materials. It is also shown that the difference between the two configurations is greater in the CNT bundles than that of the SiCNT bundles.
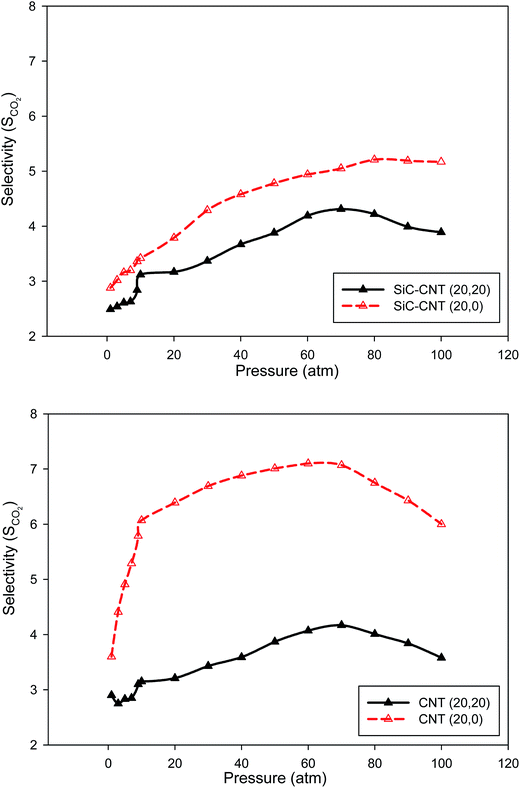 | ||
| Fig. 4 Compared between the CO2 selectivity in the armchair and zigzag SiCNT and CNT bundles at 300 K. | ||
In order to investigate the temperature effects on the CO2 selectivity, we have compared the selectivity in the armchair SiCNT and CNT bundles at different temperatures in Fig. 5. It is shown that the selectivity increases with decreasing the temperature and the greatest selectivity of CO2 occurs at 280 K. This can be due to the fact that the repulsion forces are dominant at higher temperatures. Therefore, the adsorbate–adsorbent interactions are less significant at higher temperatures and so, the adsorption and selectivity decreases. It is also shown that the CO2 selectivity is almost the same for the SiCNT and CNT bundles at different temperatures. The kind of the nanotube bundle can just affect the surface coverage. Malek and Sahimi33 also obtained the similar results for the CO2 and CH4 adsorptions in the CNTs and SiCNTs. It is also shown that the difference between the selectivity at different temperatures is greater for the CNT bundles than the SiCNT ones. For careful observations, the actual values of the Fig. 3–5 have been presented in Tables S2–S4 in the ESI,† respectively.
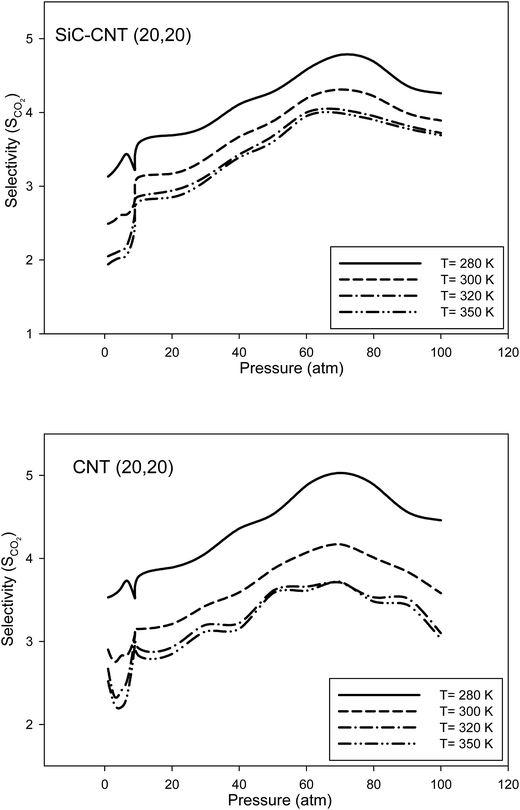 | ||
| Fig. 5 Comparison between the selectivity in the armchair SiCNT and CNT bundles at different temperatures. | ||
3.2 Diffusion coefficients
We have presented the diffusion coefficients of CO2 and CH4 as a function of the total bulk pressure in the different armchair SiCNT bundles at 300 K in Fig. 6.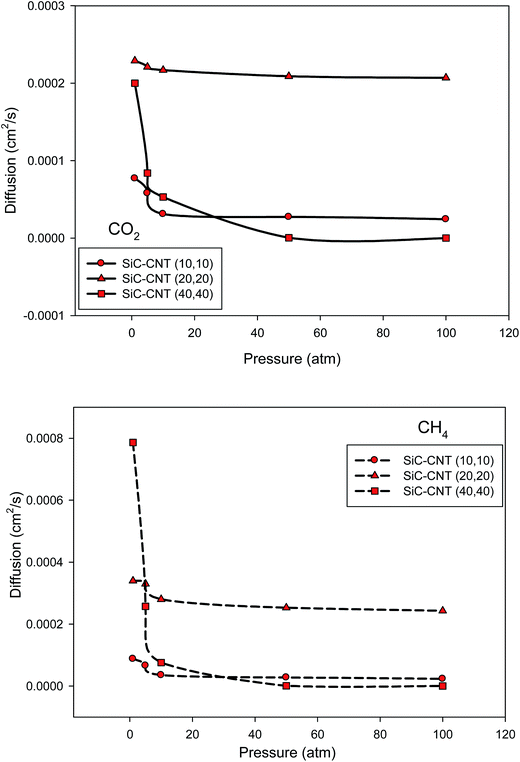 | ||
| Fig. 6 Comparison between the diffusion coefficients of CO2 and CH4 as a function of the total pressure in the different armchair SiCNT bundles at 300 K (the average anticipated error is 5%). | ||
At low pressures, the diffusivities of the two gases decrease sharply but, the rate of decrease in the diffusion decreases markedly at higher pressures. This result is in agreement with our selectivity results. In the other words, the sharp decrease of the diffusion coefficient of the molecules at low pressures is caused by a sharp increase in the amount of adsorption. This is expected since it has been shown that in the low pressure limit, the adsorbate–adsorbent interactions are significant. It is also shown that the diffusion of the two gases remain nearly constant at high pressures. Although the diffusion coefficients of the two gases increase with increasing the pore diameter from 1.35 nm (SiCNT (10,10)) to 2.71 nm (SiCNT (20,20)), but they has different behavior in the SiCNT (40,40). Initially, the diffusions of CO2 and CH4 in the SiCNT (40,40) are greater than those of the SiCNT (10,10) and SiCNT (20,20), respectively but they sharply decrease at higher pressures. It is also shown that the diffusion coefficient of CH4 is always greater than that of CO2 which can be due to the smaller interaction between the CH4 and the pore walls which leads to faster dynamics of CH4 molecules than CO2 molecules inside the SiCNT bundles.
In order to compare our diffusion results in the SiCNT bundles with the CNT bundles, we have also presented the diffusion coefficients of the two gasses in the different armchair CNT bundles at 300 K in Fig. 7. According to Fig. 6 and 7, the diffusion coefficients of the two gases in the CNT bundles are always greater than those of the SiCNT bundles which is due to the different interactions and orientation between the gas molecules and the nanotube walls.34,48 It is also shown that the diffusion coefficients of the two gases increase with increasing the pore diameter. We have also compared the diffusion coefficients of the two gases in the armchair and zigzag SiCNT and CNT bundles at 300 K in Fig. 8. According to this figure, the both gases exhibit a decrease in the diffusion coefficients vs. pressure and the slope of these changes is greater for the zigzag SiCNTs. It is also shown that the diffusion coefficients of the both gases in the zigzag SiCNTs are smaller than those values in the armchair nanotubes at high pressures. It is also shown in Fig. 8 that the diffusion coefficients of the both gases in the zigzag CNT are smaller than those of the armchair CNT at all pressure values. These results are in agreement with our selectivity and adsorption results. In the other words, the molecules have greater interactions with the zigzag wall (and so they have slower diffusions) than the armchair wall. These results indicate that the topologies of the SiCNT and CNT bundles (zigzag and armchair configurations) have significant influences on the diffusion coefficients of the gases. Malek and Sahimi33 also reported that the nanotube chirality has strong effect on the self-diffusivities of different gases in the SiCNT.
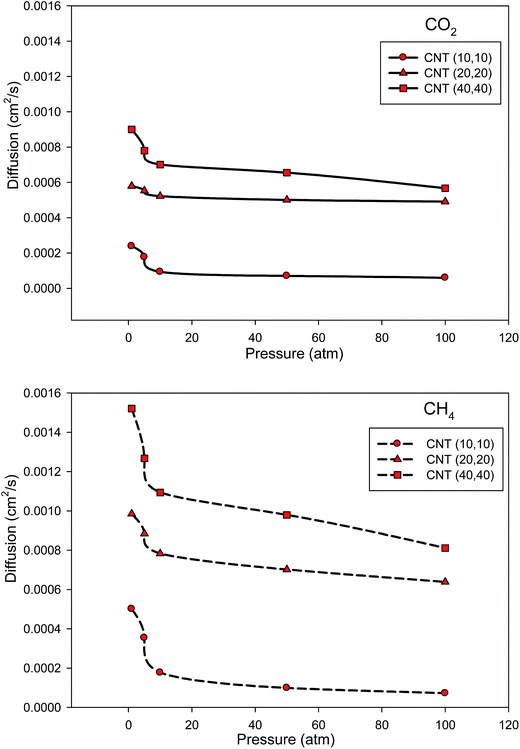 | ||
| Fig. 7 Comparison between the diffusion coefficients of CO2 and CH4 in the different armchair CNT bundles at 300 K (the average anticipated error is 5%). | ||
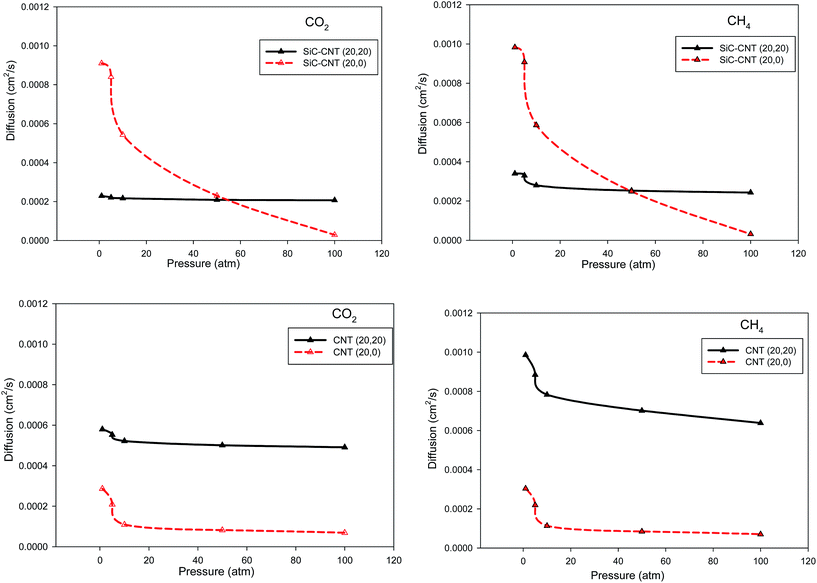 | ||
| Fig. 8 Comparison between the diffusion coefficients of CO2 and CH4 in the armchair and zigzag SiCNT and CNT bundles at 300 K (the average anticipated error is 5%). | ||
In order to investigate the temperature effects on the diffusion coefficients of the two gases, we have compared the diffusions of the two gases in the armchair SiCNT and CNT bundles at different temperatures in Fig. 9. It is expected and shown that the diffusion coefficients increase with the temperature in the both CNT and SiCNT bundles and for the both gases.
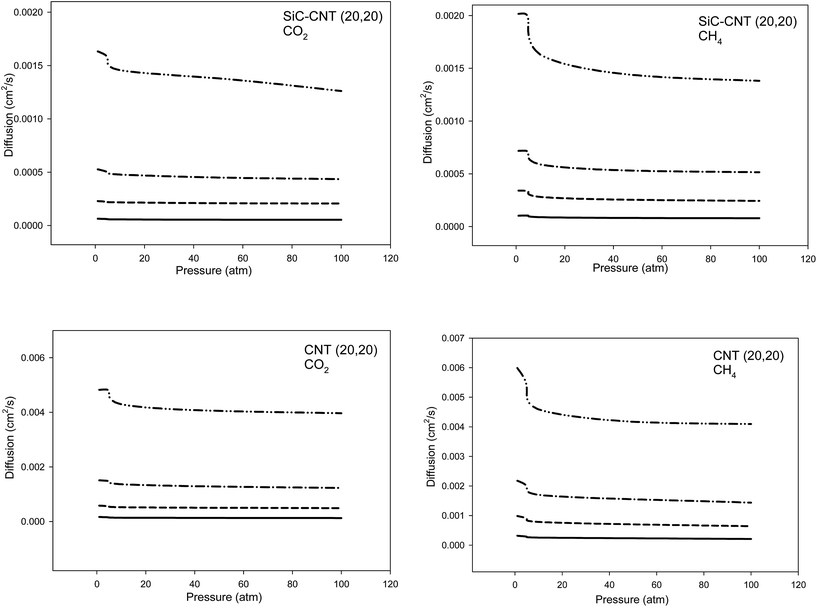 | ||
| Fig. 9 Comparison between the diffusion coefficients of CO2 and CH4 in the armchair SiCNT and CNT bundles at different temperatures (the average anticipated error is 5%). | ||
4. Conclusions
We have performed the MD simulations to investigate the separation and diffusion of a CO2 + CH4 equimolar mixture using the SiCNT and CNT bundles as potential molecular sieves with the different radii and chiralities. The following important results have been also summarized:(1) The carbon dioxide molecules are preferentially adsorbed over the methane ones which is due to the stronger interaction of CO2 molecules with the pore walls.
(2) The SCO2 is relatively lower in the larger nanotube bundles which may be due to the weaker adsorbate–adsorbent interactions in the larger bundles than the smaller ones.
(3) The smaller CNTs adsorb more CO2 than the SiCNT, but the larger CNTs adsorb less carbon dioxide than the SiCNTs which may be due to the different orientation of the gas molecules toward various binding sites.
(4) The selectivity isotherm in the zigzag nanotube exhibits sharp increase in the adsorption which may be due to the stronger interactions between the CO2 molecules and the zigzag walls than the armchair wall.
(5) The selectivity increases with decreasing the temperature which can be due to the fact that the repulsion forces are dominant at higher temperatures and so, the adsorbate–adsorbent interactions are less significant at higher temperatures.
(6) The CO2 selectivity is almost the same for the both SiCNT and CNT bundles at different temperatures.
(7) At low pressures, the diffusivities of the two gases decrease sharply with increasing pressure. This result is in agreement with our selectivity results.
(8) The diffusion coefficients of the two gases usually increase with increasing the pore diameter.
(9) The diffusion coefficient of CH4 is always greater than that of CO2 which can be due to the smaller interaction between the CH4 and the pore walls.
(10) The diffusion coefficients of the two gases in the CNT bundles are always greater than those of the SiCNT bundles.
(11) The diffusion coefficients of the both gases in the zigzag CNT are smaller than those of the armchair CNT at all pressure values which is due to the greater interactions of the molecules with the zigzag wall.
References
- I. Skarmoutsos, G. Tamiolakis and G. E. Froudakis, Phys. Chem. Chem. Phys., 2014, 16, 876–879 RSC
.
- S. Li and C. Q. Fan, Ind. Eng. Chem. Res., 2010, 49, 4399–4404 CrossRef CAS
.
- M. P. Bernal, J. Coronas, M. Menéndez and J. Santamara, AIChE J., 2004, 50, 127–135 CrossRef CAS
.
- J. C. Palmer, J. D. Moore, T. J. Roussel, J. K. Brennanb and K. E. Gubbinsa, Phys. Chem. Chem. Phys., 2011, 13, 3985–3996 RSC
.
- D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed
.
- C. H. Yu, C. H. Huang and C. S. Tan, Aerosol Air Qual. Res., 2012, 12, 745–769 CAS
.
- F. Karadas, M. Atilhan and S. Aparicio, Energy Fuels, 2010, 24, 5817–5828 CrossRef CAS
.
- D. Aaron and C. Tsouris, Sep. Sci. Technol., 2005, 40, 321–348 CrossRef CAS
.
- Y. S. Bae and R. Q. Snurr, Angew. Chem., Int. Ed., 2011, 50, 11586–11596 CrossRef CAS PubMed
.
- I. Skarmoutsos, G. Tamiolakis and G. E. Froudakis, J. Phys. Chem. C, 2013, 117, 19373–19381 CAS
.
- D. Mantzalis, N. Asproulis and D. Drikakis, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2011, 84, 066304 CrossRef PubMed
.
- X. Peng, D. Cao and W. Wang, Chem. Eng. Sci., 2011, 66, 2266–2276 CrossRef CAS
.
- S. M. Sanip, A. F. Ismail, P. S. Goh, T. Soga, M. Tanemura and H. Yasuhiko, Sep. Purif. Technol., 2011, 78, 208–213 CrossRef CAS
.
- P. Kowalczyk, Phys. Chem. Chem. Phys., 2012, 14, 2784–2790 RSC
.
- H. Yi, F. Li, P. Ning, X. Tang, J. Peng, Y. Li and H. Deng, Chem. Eng. J., 2013, 215, 635–642 CrossRef
.
- P. Billemont, B. Coasne and G. De Weireld, Langmuir, 2013, 29, 3328–3338 CrossRef CAS PubMed
.
- M. Palomino, A. Corma, F. Rey and S. Valencia, Langmuir, 2010, 26, 1910–1917 CrossRef CAS PubMed
.
- R. Krishna and J. M. van Baten, Chem. Eng. J., 2007, 133, 121–131 CrossRef CAS
.
- J. M. Leyssale, G. K. Papadopoulos and D. N. Theodorou, J. Phys. Chem. B, 2006, 110, 22742–22753 CrossRef CAS PubMed
.
- S. Li, G. Alvarado, R. D. Noble and J. L. Falconer, J. Membr. Sci., 2005, 251, 59–66 CrossRef CAS
.
- S. J. Shilton, A. F. Ismail, P. J. Gough, I. R. Dunkin and S. L. Gallivan, Polymer, 1997, 38, 2215–2220 CrossRef CAS
.
- N. V. Blinova and F. Svec, J. Membr. Sci., 2012, 423, 514–521 CrossRef
.
- P. Uchytil, J. Schauer, R. Petrychkovych, K. Setnickova and S. Y. Suen, J. Membr. Sci., 2011, 383, 262–271 CrossRef CAS
.
- K. Sumida, D. L. Rogow, J. A. Mason, T. M. McDonald, E. D. Bloch, Z. R. Herm, T. H. Bae and J. R. Long, Chem. Rev., 2012, 112, 724–781 CrossRef CAS PubMed
.
- F. A. Cabrales-Navarro, J. L. Gomez-Ballesteros and P. B. Balbuena, J. Membr. Sci., 2013, 428, 241–250 CrossRef CAS
.
- R. Eguchi, S. Uchida and N. Mizuno, J. Phys. Chem. C, 2012, 116, 16105–16110 CAS
.
- S. Basu, A. Cano-Odena and I. F. J. Vankelecom, Sep. Purif. Technol., 2011, 81, 31–40 CrossRef CAS
.
- P. Mishra, S. Mekala, F. Dreisbach, B. Mandal and S. Gummaa, Sep. Purif. Technol., 2012, 94, 124–130 CrossRef CAS
.
- Zh. Zhang, Zh. Li and J. Li, Langmuir, 2012, 28, 12122–12133 CrossRef CAS PubMed
.
- Y. Zhu, J. Zhou, J. Hu, H. Liu and Y. Hu, Chin. J. Chem. Eng., 2011, 19, 709–716 CrossRef CAS
.
- H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 8875–8883 CrossRef CAS PubMed
.
- R. Babarao and J. Jiang, Langmuir, 2008, 24, 5474–5484 CrossRef CAS PubMed
.
- K. Malek and M. Sahimi, J. Chem. Phys., 2010, 132, 014310 CrossRef PubMed
.
- M. A. Baghernia and M. Shadman, Int. Nano Lett., 2014, 4, 95 CrossRef
.
- S. Sircar, T. C. Golden and M. B. Rao, Carbon, 1996, 34, 1–12 CrossRef CAS
.
- I. K. Nikolaidis, G. C. Boulougouris, L. D. Peristeras and I. G. Economou, Ind. Eng. Chem. Res., 2016, 55, 6213–6226 CrossRef CAS
.
- F. Huarte-Larrañaga and M. Alberti, Chem. Phys. Lett., 2007, 445, 227 CrossRef
.
- V. R. Cervellera, M. Alberti and F. Huarte-Larrañaga, Int. J. Quantum Chem., 2008, 108, 1714 CrossRef CAS
.
- S. Vela and F. Huarte-Larrañaga, Carbon, 2011, 49, 4544 CrossRef CAS
.
- H. J. C. Berendsen, J. P. M. Postma, W. F. van Gunsteren, A. Dinola and J. R. Haak, J. Chem. Phys., 1984, 81, 3684 CrossRef CAS
.
- W. Smith and I. T. Todorov, A short description of DL_POLY, Mol. Simul., 2006, 32, 935 CrossRef CAS
.
- M. G. Martin and J. I. Siepmann, J. Phys. Chem. B, 1998, 102, 2569 CrossRef CAS
.
- J. J. Potoff and J. I. Siepmann, AIChE J., 2001, 47, 1676 CrossRef CAS
.
- W. F. van Gunsteren and H. J. C. Berendsen, Angew. Chem., 1990, 29, 992 Search PubMed
.
- S. Nóse, J. Phys.: Condens. Matter, 1990, 2, 115–119 CrossRef
; W. G. Hoover, Phys. Rev. A, 1985, 31, 1695–1697 CrossRef
.
- G. Mpourmpakis, G. E. Froudakis, G. P. Lithoxoos and J. Samios, J. Chem. Phys., 2007, 126, 144704 CrossRef PubMed
.
- L. Huang, L. Zhang, Q. Shao, L. Lu, X. Lu, S. Jiang and W. Shen, J. Phys. Chem. C, 2007, 111, 11912–11920 CAS
.
- G. Mpourmpakis, G. E. Froudakis, G. P. Lithoxoos and J. Samios, Nano Lett., 2006, 6, 1581–1583 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra16672e |
| This journal is © The Royal Society of Chemistry 2016 |