Sphere-like CoS with nanostructures as peroxidase mimics for colorimetric determination of H2O2 and mercury ions†
Haiguan Yang,
Junqi Zha,
Peng Zhang,
Yuhao Xiong,
Linjing Su and
Fanggui Ye*
State Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources, College of Chemistry and Pharmaceutical Science of Guangxi Normal University, Guilin 541004, P. R. China. E-mail: fangguiye@163.com; Fax: +86-773-5832294; Tel: +86-773-5856104
First published on 8th July 2016
Abstract
CoS, which was prepared using a facile solvothermal method, and characterized using various analytical techniques, was demonstrated for the first time to exhibit intrinsic peroxidase-like activity. CoS catalyzed the oxidation of the peroxidase substrate 3,3′,5,5′-tetramethylbenzidine in the presence of H2O2 rendering the reaction solution blue, which is the principle used for H2O2 sensing. The mechanism of the CoS catalysis was also investigated. Under optimal conditions, the linearity of H2O2 detection ranged from 0.05 to 0.8 mM (R2 = 0.9965). More importantly, using the reducibility of glutathione and the strong affinity to thiol compounds exhibited by mercury ions (Hg2+), a novel “off–on” colorimetric sensor for Hg2+ determination was designed using CoS as a peroxidase mimics. The analytical platform for Hg2+ determination was developed, ranging from 0.25 to 3 μM (R2 = 0.9965). The limit of detection was 0.1 μM. CoS exhibited several advantageous features including good chemical stability, ease of preparation, simple purification, and excellent reproducibility.
Introduction
Natural enzymes have received wide ranging attention for more than 200 years, and play an important role in biochemistry and biosensors, because they exhibit excellent substrate specificity and efficiency under mild conditions. However, there are some drawbacks limiting their practical application, such as low stability and declining catalytic activity under harsh conditions. Moreover, their preparation and handling are expensive and time-consuming.1 Therefore, in recent years, much effort has been dedicated to the development of enzyme mimics with catalytic activity similar to that of natural enzymes, such as superoxide dismutase, catalase, peroxidase, oxidase, esterase, nuclease, ferroxidase, phosphatase, and protease.2In particular, the advantages of nanomaterial-based enzyme mimics, have received much attention, such as, their large surface-to-volume ratio, ease of preparation, and relatively simple procedures for storage and separation. Nanomaterial-based enzyme mimics have been studied extensively for various applications, including biosensors, immunoassays, and sensing.3 Fe3O4 magnetic nanoparticles were first found to possess intrinsic peroxidase-like activity by the Yan's group.4 Since then, various nanomaterials such as metal–organic frameworks (MIL-53(Fe),5 MIL-68,6 MIL-100,6 and HKUST-1 (ref. 7)), metallic oxide nanoparticles (CeO2,8 V2O5,9 CuO,10 MnO2,11 and Co3O4 (ref. 12)), metallic and bimetallic nanoparticles (Au,13 Ag,14 Pt,15 and Au@Pt),16 carbon-based nanomaterials (graphene oxide,17 carbon nanotubes,18 and carbon dots19), bimetallic oxides (ZnFe2O4,20 CoFe2O4,21 and FeWO4 (ref. 22)) have been reported to exhibit intrinsic enzyme mimetic activity and have been used for colorimetric sensing of H2O2, Hg2+, glucose, and other species.23
In recent years, metal sulfide nanostructures have attracted much attention due to their excellent optical and electronic properties, and wide ranging of applications in catalysis.24 For example, FeS,25 CdS,26 and CuS27 have all been shown to possess peroxidase-like activity. Cobalt sulfides are of interest as semiconductor materials, due to their advanced electronic properties, with applications in energy storage, lithium-ion batteries, solar cells and more.28 The simplest cobalt sulfide, CoS, has been investigated in many fields due to its advantages. Qu et al.29 prepared a plate-like CoS electrocatalyst for use in the hydrogen evolution reaction. Wang et al.30 developed a CoS spindle as an efficient counter electrode for dye-sensitized solar cells. Jiao et al.31 studied the electrochemical properties of flower-like CoS in lithium ion batteries. Long et al.32 found that electrodeposited Co-S film catalyzed both electrochemical and photoelectrochemical hydrogen generation from water. Li et al.33 reported that CoS nanowires were successfully synthesized and used in supercapacitors. However, to our knowledge, there have been no investigations into the peroxidase-like activity of CoS in the enzyme mimics field. To exploit a new application of CoS is attractive prospect in nanoscience and nanotechnology.
In this work, CoS was found to exhibit intrinsic peroxidase mimetic activity for the first time. A facile and green solvothermal method was used for CoS preparation. The as-synthesized CoS catalyzed the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) in the presence of H2O2 to produce a blue color change in the reaction solution. Glutathione (GSH) successfully caused a decline in blue color generation due to its strong radical restoration ability. Mercury ions (Hg2+) exhibited a strong affinity for the thiolated compounds. Based-on these phenomena, using CoS as a peroxidase mimic offered a simple colorimetric sensor for use in H2O2 detection, and a novel “off–on” colorimetric sensor designed for Hg2+ determination. CoS demonstrated a great potential for applications in biosensors, medical diagnosis, and environmental conservation.
Experimental
Chemicals and materials
All chemicals were obtained from commercial sources as analytical grade reagents (unless otherwise stated) and used without further purification. TMB, ABTS and OPD were purchased from TCI (Shanghai, China). Calcium chloride (CaCl2), acetic acid (HAc), sodium chloride (NaCl), H2O2 (30 wt%), lead chloride (PbCl2), mercury chloride (HgCl2), zinc chloride (ZnCl2), and sodium acetate (NaAc) were obtained from Shantou Xilong Chemical Co. Ltd. (Guangdong, China). Nickel nitrate (Ni(NO3)2), chromic nitrate (Cr(NO3)3), ferrous nitrate (Fe(NO3)2), cadmium chloride (CdCl2), tert-butyl alcohol, silver nitrate (AgNO3), magnesium sulfate (MgSO4), and ammonium chloride (NH4Cl) were purchased from Aladdin Chemistry Co. Ltd (Shanghai, China). HRP and GSH were from Sigma-Aldrich (St. Louis, MO, USA). Ultrapure water was produced by a Millipore purification system (Bedford, MA, USA) and used to prepare all aqueous solutions.Apparatus
Absorption spectra were obtained using a model Cary 60 spectrophotometer (Agilent, USA). Powder X-ray diffraction (XRD) patterns for CoS were obtained using a D/max 2550 VB/PC diffractometer (Rigaku, Japan) using Cu Kα radiation (λ = 0.15418 nm) from 5 to 90°. X-ray photoelectron spectroscopy (XPS) was performed on a Thermo-ESCALAB 250XI (Thermo, USA) instrument with non-monochromated Al Kα 1486.6 eV radiation. Energy corrections were performed using the C 1s peak (284.7) as reference. Scanning electron microscopy (SEM) images were recorded on a field-emission microscope (JSM 7600F). Transmission electron microscopy (TEM) was performed using a Tecnai G20 (FEI, USA). The inductively coupled plasma mass spectrometry (ICP-MS) was recorded by Flexar/NexlON300X (PerkinElmer, USA). High-resolution transmission electron microscope (HRTEM) was obtained using a FEI Fecnai G2 F20 S-Twin transmission electron microscope, operating at an accelerating voltage of 200 kV.Synthesis of CoS
CoS was synthesized according to the procedure of Wu et al.28 with slight modifications. Typically, the cobalt source was cobalt chloride hexahydrate (CoCl2·6H2O) and the sulfur source was thiourea (CN2H4S). The mixed solution was prepared by dissolving CoCl2·6H2O (594.8 mg) into a mixture solution of ultrapure water (20 mL) and ethylene glycol (5 mL), and then thiourea (761.2 mg) was added. After stirring for 10 min, the mixed solution was transferred into a 50 mL Teflon-lined stainless steel autoclave, and heated for 15 h at 180 °C. Finally, the autoclave was cooled to room temperature, and the black powder was collected by centrifugation and washed several times with ultrapure water and ethanol to remove unreacted reagents. The black powder of CoS was dried for 12 h at 50 °C under vacuum conditions.Mimetic peroxidase activity tests
All reactions were monitored in time scan mode at 652 nm using a Cary 60 spectrophotometer. Kinetic measurements were implemented by monitoring the absorbance change at 652 nm. Typical catalytic tests: used 0.5 mg mL−1 CoS or 1.25 ng mL−1 HRP, 0.15 mM TMB, and 20 mM H2O2 for CoS, or 2 mM H2O2 for HRP, as the substrates in a reaction volume of 2 mL. Kinetic constants were calculated using the Lineweaver–Burk plots of the double reciprocal of the Michaelis–Menten equation 1/ν = Vmax[S]/(Km + [S]), where ν is the initial velocity, Vmax is the maximum reaction velocity, [S] is the concentration of the substrate, and Km is the Michaelis constant.4Determination of H2O2 and Hg2+ using CoS
H2O2 determination was conducted as follows: CoS (0.5 mg mL−1), TMB (0.15 mM), and HAc–NaAc buffer (1.8 mL, 0.2 M, pH 3.5) were added to 5 mL centrifuge tube. Then H2O2 (100 μL) was added at different concentrations. Subsequently, the solutions were incubated for 10 min at room temperature (25 °C). The absorption spectra of the solutions were measured using a model Cary 60 spectrophotometer.Hg2+ determination was performed as follows: at room temperature, Hg2+ solutions (50 μL) at different concentrations, and GSH (0.1 mM) were added to NaAc–HAc buffer (1.7 mL, 0.2 M, pH 3.5). After incubation for 5 min, TMB (0.15 mM) and CoS (0.5 mg mL−1) were add. The final volume of the mixed solution was 2 mL, which was then incubated for another 10 min. Lastly, a model Cary 60 spectrophotometer was used to measure the absorption spectra of the mixed solutions.
Determination of real samples
Li River water and tap water samples were analyzed as real samples without further treatment. Li River water and tap water samples (15 mL each) were diluted to 30 mL with acetate buffer (0.4 M, pH 3.5). A 3.5 mL aliquot of these solutions was added to a 5 mL centrifugal tube, and spiked with GSH (0.1 mM) and Hg2+ solutions at various concentrations. After incubation for 5 min, TMB (0.15 mM) and CoS (0.5 mg mL−1) were added. The final volume was 4 mL, which was then incubated for another 10 min at 25 °C. The mixed solutions were analyzed using the proposed method and the percentage recovery values were obtained.Results and discussion
Characterization of CoS
XRD patterns of the as-prepared CoS were shown in Fig. 1a. As can be seen, the main diffraction peaks appeared at 2θ values of 30.6°, 35.3°, 46.9°, 54.4°, and 74.6°, which coincide with the (100), (101), (102), (110), and (202) surfaces of hexagonal CoS (JCPDS card No. 65-3418, cell = 3.368 × 5.17, α = 90°, β = 90°, γ = 120°). No impure peaks were observed, which proved that the product was highly crystalline and pure. Significantly, the crystal structure of CoS remained very well intact after catalytic reaction, five recycle sensing H2O2 and five recycle sensing Hg2+. A typical Raman scattering spectrum was used to further study the CoS structure (Fig. 1b). The four remarkable peaks at 476, 520, 606, and 685 cm−1 corresponded with the Eg, F12g, F22g, and A1g modes of CoS, respectively.34X-ray photoelectron spectra (XPS) of CoS, as well as the CoS Co 2p, and S 2p spectra were studied (Fig. 2). As shown in Fig. 2a, all peaks could be attributed to Co, S, C, and O elements. The appearance of the O element peak (531.7 and 533.0 eV) in the sample might be attributed to the oxygen of the hydroxide ions and a small amount of physically adsorbed water molecules.35 There are two strong peaks at about 793.1 and 778.1 eV with a weak peak at about 780.2 eV, which are ascribed to the Co 2p1/2 and Co 2p3/2 binding energies (Fig. 2b), these results corresponds to Co2+ of CoS.36–40 In Fig. 2c, peaks could be observed at 161.5 and 162.6 eV in the region of S 2p due to the S 2p3/2 and S 2p1/2 binding energies, which corresponds to S−2 of CoS.38–40 The observation of a weak peak at 168.5 eV is associated with sulfur oxides.41,42
 | ||
| Fig. 2 (a) XPS analysis of as-prepared CoS, (b) and (c) show narrow range XPS scans for the Co and S signals, respectively. | ||
The energy dispersive X-ray spectrometry (EDS) spectrum and elemental mapping images were shown in Fig. 3. The EDS spectrum revealed that S and Co were found in the as-prepared CoS. The atom ratio of S and Co elements in CoS was nearly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 3a). The elemental mapping images revealed that S and Co were uniformly distributed in the as-prepared CoS (Fig. 3b). Furthermore, the as-prepared sample was also investigated using inductively coupled plasma mass spectrometry (ICP-MS) (Table S1†), which indicated that the Co composition was close to the theoretical value, suggesting that CoS had been successfully synthesized.
1 (Fig. 3a). The elemental mapping images revealed that S and Co were uniformly distributed in the as-prepared CoS (Fig. 3b). Furthermore, the as-prepared sample was also investigated using inductively coupled plasma mass spectrometry (ICP-MS) (Table S1†), which indicated that the Co composition was close to the theoretical value, suggesting that CoS had been successfully synthesized.
 | ||
Fig. 3 (a) The EDS spectrum, (b) elemental mapping images of S and Co in CoS. The atom ratio of S and Co elements in CoS was around 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. 1. | ||
The CoS was investigated using scanning electron microscopy (SEM), transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) (Fig. 4). SEM images of as-prepared CoS and the CoS after sensing Hg2+ were composed of sphere-like nanostructures, with no nanoflakes on the surface (Fig. 4a–d). The CoS retains its shape after sensing Hg2+. The TEM image further demonstrated that the as-prepared CoS exhibited sphere-like nanostructures (Fig. 4e). The calculated lattice spacing were 0.252 nm and 0.298 nm, which respectively matched well with the (101) and (100) planes of the as-prepared CoS (JCPDS card No. 65-3418) (Fig. 4f).
 | ||
| Fig. 4 (a and b) The SEM images of as-prepared CoS, (c and d) the SEM images of CoS after sensing Hg2+, (e) the TEM image and (f) HRTEM of as-prepared CoS. | ||
Peroxidase-like activity of CoS
The peroxidase-like activity of CoS was investigated using the catalytic oxidation of TMB as a peroxidase substrate in the presence of H2O2. TMB is a typical chromogenic substrate. First, the peroxidase-like activity of CoS was examined. As shown in Fig. 5a, the CoS/H2O2 and CoS/TMB systems were colorless with no absorbance at 652 nm. However, the H2O2/TMB system exhibited a light blue color and very weak absorption at 652 nm. However, in the presence of CoS, the H2O2/TMB system produced a typical blue color reaction and a strong absorption peak was observed at 652 nm. H2SO4 was also used to test the catalytic activity of CoS. As shown in Fig. 5b, the absorption peak at 652 nm gradually declined with increasing H2SO4 concentration. This was similar to that observed for the peroxidase activity of natural enzymes, in which that blue color was quenched by adding various concentrations of H2SO4.4 In order to further characterize the peroxidase-like activity of CoS, ABTS and OPD were also investigated as peroxidase substrates (Fig. S1†). ABTS and OPD changed from colorless to green and orange, respectively. ABTS and OPD also had absorption maxima at 414 nm and 450 nm, respectively. These results confirmed that CoS exhibited catalytic properties. In contrast with Co3O4, the catalytic activity of CoS has rarely been studied making, this discovery particularly interesting. Moreover, the reaction rate was dependent on the reaction time and amount of CoS (Fig. S2†), further confirming the peroxidase-like activity of CoS.It was important to determine whether this catalytic activity was caused by intact CoS or cobalt ions leaching into the acidic solution. To study this, CoS was incubated in the standard NaAc–HAc buffer (0.2 M, pH 3.5) for 10 min (the reaction time needed to reach maximum catalytic activity). Then, CoS was removed by centrifugal separation (14000 rpm, 2 min). The catalytic activity of CoS was compared with that in the leaching solution under the same conditions. As shown in Fig. 6a, CoS had good activity, but the leaching solution had no activity. This result indicated that the catalytic activity originated from intact CoS. In order to determine the CoS catalytic mechanism, tert-butyl alcohol was applied as a typical ˙OH radical capture reagent to the CoS/TMB/H2O2 reaction system (Fig. 6b). tert-Butyl alcohol can react rapidly with ˙OH and terminate radical chain reactions by generating inert intermediate radicals.43 Experimental results revealed that the absorbance intensity gradually decreased as tert-butyl alcohol concentration increased from 0 to 400 mg mL−1, which indicated that the peroxidase-like activity of CoS in the catalytic oxidation of TMB in the presence of H2O2 originated from H2O2 decomposition generating the ˙OH radicals.
 | ||
| Fig. 6 (a) Time dependent absorbance evolution at 652 nm for TMB oxidation system in the presence of CoS and leaching solution. (b) Effect of tert-butyl alcohol concentration on TMB oxidation. | ||
Optimization of experimental conditions
The catalytic activity of CoS was similar to that of HRP, and was dependent on pH, temperature and H2O2 concentration. As shown in Fig. S3,† different pH values (2.5–6), the temperatures (20–60 °C), and H2O2 concentrations (0.1–200 mM) were investigated. These results were compared with those of HRP under the same condition. The optimum pH and temperature for CoS were 3.5 and 25 °C, respectively. And the best pH and temperature for HRP were 4 and 35 °C, respectively. These values for CoS and HRP were similar, therefore, pH 3.5 and 25 °C were selected as the optimal conditions for studying CoS catalytic activity. As shown in Fig. S3c,† CoS needed a higher concentration of H2O2 than HRP to reach maximum catalytic activity. Nevertheless, further increasing H2O2 concentration restricted the catalytic activities of both CoS and HRP. To accurately achieve maximum CoS activity, the reaction time and amount of CoS were also investigated. Relative activity gradually increased with reaction time, reaching a maximum value at 10 min, which was used in all subsequent tests (Fig. S3e†). The amount of CoS also affected catalytic activity. The relative activity rapidly increased, and then decreased, as CoS concentration was gradually increased (Fig. S3d†). A CoS concentration of 0.5 mg mL−1 was chosen as the standard condition for subsequent analysis.Kinetic analysis of CoS as peroxidase mimics
Under the optimal conditions, the mechanism of catalytic activity and the kinetic parameters of CoS were investigated using enzyme kinetics theory and methods with H2O2 and TMB as substrates. Within a certain range of substrate concentrations, typical Michaelis–Menten curves were obtained for CoS and HRP (Fig. 7). Kinetic parameters were acquired by changing the concentration of one substrate while the other remained the same. The Michaelis–Menten constant (Km) and the Maximum initial velocity (Vmax) were calculated according to the Lineweaver–Burk plot.4 The kinetic parameters of CoS and HRP are listed in Table S2.† The Km value for CoS with TMB as the substrate was 0.41 mM, which was slightly lower than that of HRP. Moreover, the Vmax value for CoS was higher than that of HRP. These results suggested that CoS had a higher catalytic activity in the reaction with TMB than HRP. The Km value of CoS with H2O2 as a substrate was 7.15, which was higher than that of HRP. This result revealed that CoS had a lower affinity for H2O2 than HRP. This result agreed with the observation that a higher concentration of H2O2 was required to obtain maximum catalytic activity from CoS. The kinetic parameters of CoS also compared to other metal oxide and metal sulphide nanostructures (Table S2†). The kinetic parameters of CoS showed a very comparable affinity for TMB and H2O2.Determination of H2O2 using CoS–TMB system
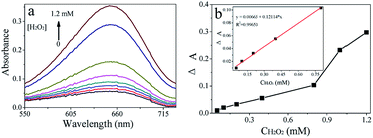
A simple and rapid colorimetric method for the determination of H2O2 was developed based on the catalytic activity of CoS (Fig. 9a). The dependency of CoS catalytic activity on H2O2 concentration was discussed above, suggesting that the CoS–TMB system could be applied to H2O2 determination. Under optimal conditions, the changes in absorbance curves at different H2O2 concentrations were investigated (Fig. 8a). The concentration of H2O2 ranged from 0.05 to 1.2 mM. The linearity ranged of 0.05 to 0.8 mM (R2 = 0.9965) (Fig. 8b), and the limit of detection for H2O2 was estimated to be 0.02 mM using the formula, S/N = ((averagesample − averageblank)/SDblank). Sample concentrations consistent with 3 < S/N < 5 condition were defined as the limits of detection.44 The linearity and limits of detection of this colorimetric method and those of other nanomaterials-based colorimetric methods are listed in Table S3.†Mechanism and colorimetric determination of Hg2+ in aqueous solution
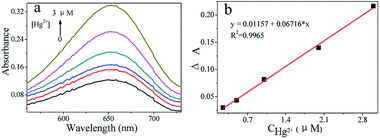
The production of TMB cation radical (TMB+) was detected in the three systems (CoS, CoS–GSH, and CoS–GSH–Hg2+) at 652 nm under optimal reaction conditions. As shown in Fig. S4,† the color changed from deep blue to a very light blue, while the absorption peak was decreased dramatically in the presence of GSH. However, the color returned to deep blue and the absorption peak reappeared, in the presence of Hg2+. Based on CoS catalytic activity, the reduction properties of GSH, and the strong affinity of Hg2+ for thiol compounds, a simple and novel “off–on” colorimetric method was developed for Hg2+ detection. The sensing method in this work is described in Fig. 9a. The sensor was in the “off” state, when GSH was added to the CoS system, because GSH could restore TMB+ to the original colorless TMB molecule by its thiol functionality. These results were similar to some reported reactions,45,46 the reaction equations were shown in Fig. 9b. When GSH bonded to Hg2+, the sensor was in the “on” state due to the strong affinity between Hg2+ and GSH.45 This phenomenon was believed to be due to the Hg2+–thiol complexes, which had high stability constants,47,48 and the reaction equations were shown in Fig. 9c. The changes in absorbance curves were studied at various Hg2+ concentrations (Fig. 10). The linearity ranged from 0.25 to 3 μM and the limit of detection was estimated to be 0.1 μM based on the same formula as previously. The results of Hg2+ detection using this and previously reported nanomaterials-base methods are summarized in Table S4.† Compared to other nanomaterial-based colorimetric detection methods for Hg2+, the above colorimetric method showed a very comparable detection limit. | ||
| Fig. 9 (a) Schematic illustration of colorimetric sensing of H2O2 and Hg2+ using CoS catalyzed reactions, (b and c) the reaction equations illustration of colorimetric sensing process. | ||
Selectivity of the Hg2+ colorimetric determination method
To test the selectivity of Hg2+ determination control experiments were performed under standard conditions using Na+, Cr3+, NH4+, Zn2+, Mg2+, Ca2+, Ni2+, Fe3+, Cd2+, and Pb2+. These ions could potentially disturb Hg2+ detection. As shown in Fig. 11, there was no apparent signal interference under the same Hg2+ concentration (3 μM). Therefore, ion interference did not influence Hg2+ determination. | ||
| Fig. 11 Detected selectivities for Hg2+ determination with other ions at the same concentration based-on CoS peroxidase-like activity. | ||
Determination of Hg2+ in real samples
To test the feasibility of the above colorimetric approach, two real water samples were tested under optimal experiment conditions. The resulting detection and recovery values are listed in Table 1, with Hg2+ recovery values at the three spiked levels ranged from 85.94 to 106.04%. The relative standard deviation (RSD) values of the measurements were obtained at each concentration level. These findings clearly indicated that this colorimetric sensor could be applied to detection in real samples.Reutilization assays
The reutilization of CoS was studied under the optimal conditions. The blue colored solutions were detected using a spectrophotometer at 652 nm after 10 min reacting with CoS. After each detection, CoS was separated by centrifugation (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 2 min) and washed several times with water and ethanol, respectively. As shown in Fig. 12, there was not obvious decline in CoS catalytic activity after five cycles, indicating that CoS was highly catalytically stable and reproducible.
000 rpm, 2 min) and washed several times with water and ethanol, respectively. As shown in Fig. 12, there was not obvious decline in CoS catalytic activity after five cycles, indicating that CoS was highly catalytically stable and reproducible.
Conclusions
In summary, CoS was prepared using a simple solvothermal method. The catalytic activity of CoS was investigated for the first time and similar to HRP, was shown to be dependent on pH, temperature, and H2O2 concentration. The catalysis was shown to coincide with typical Michaelis–Menten kinetics. CoS has several advantages, such as low cost, simple preparation, good stability, and unexceptionable reproducibility. CoS catalyzed the oxidation of TMB to generate a color-change reaction with H2O2. GSH and Hg2+ possessed a strong radical restoration ability and strong affinity for thiolated compounds, respectively. Based-on these findings, a facile and cheap method for H2O2 detection, and a novel “off–on” colorimetric sensor for the determination of Hg2+ were developed. The “off–on” colorimetric sensor was also used for the determination of real samples.Acknowledgements
The financial support from the National Natural Science Foundation of China (21365005), Guangxi Natural Science Foundation of China (2014GXNSFGA118002) and Guangxi Pharmaceutical Industry Talent Highland Project (1414) is gratefully acknowledged.Notes and references
- W. Chen, J. Chen, Y.-B. Feng, L. Hong, Q.-Y. Chen, L.-F. Wu, X.-H. Lin and X.-H. Xia, Analyst, 2012, 137, 1706–1712 RSC.
- X. Wang, Y. Hu and H. Wei, Inorg. Chem. Front., 2016, 3, 41–60 RSC.
- L. Su, W. Qin, H. Zhang, Z. U. Rahman, C. Ren, S. Ma and X. Chen, Biosens. Bioelectron., 2015, 63, 384–391 CrossRef CAS PubMed.
- L. Z. Gao, J. Zhuang, L. Nie, J. B. Zhang, Y. Zhang, N. Gu, T. H. Wang, J. Feng, D. L. Yang, S. Perrett and X. Y. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- L. Ai, L. Li, C. Zhang, J. Fu and J. Jiang, Chem.–Eur. J., 2013, 19, 15105–15108 CrossRef CAS PubMed.
- J.-W. Zhang, H.-T. Zhang, Z.-Y. Du, X. Wang, S.-H. Yu and H.-L. Jiang, Chem. Commun., 2014, 50, 1092–1094 RSC.
- H. Tan, Q. Li, Z. Zhou, C. Ma, Y. Song, F. Xu and L. Wang, Anal. Chim. Acta, 2015, 856, 90–95 CrossRef CAS PubMed.
- A. Asati, S. Santra, C. Kaittanis, S. Nath and J. M. Perez, Angew. Chem., Int. Ed., 2009, 48, 2308–2312 CrossRef CAS PubMed.
- R. André, F. Natálio, M. Humanes, J. Leppin, K. Heinze, R. Wever, H.-C. Schröder, W. E. G. Müller and W. Tremel, Adv. Funct. Mater., 2011, 21, 501–509 CrossRef.
- W. Chen, J. Chen, A.-L. Liu, L.-M. Wang, G.-W. Li and X.-H. Lin, ChemCatChem, 2011, 3, 1151–1154 CrossRef CAS.
- Y. Wan, P. Qi, D. Zhang, J. Wu and Y. Wang, Biosens. Bioelectron., 2012, 33, 69–74 CrossRef CAS PubMed.
- J. Yin, H. Cao and Y. Lu, J. Mater. Chem., 2012, 22, 527–534 RSC.
- Y. J. Long, Y. F. Li, Y. Liu, J. J. Zheng, J. Tang and C. Z. Huang, Chem. Commun., 2011, 47, 11939–11941 RSC.
- H. Jiang, Z. Chen, H. Cao and Y. Huang, Analyst, 2012, 137, 5560–5564 RSC.
- L. Chen, N. Wang, X. Wang and S. Ai, Microchim. Acta, 2013, 180, 1517–1522 CrossRef CAS.
- J. Liu, X. Hu, S. Hou, T. Wen, W. Liu, X. Zhu, J.-J. Yin and X. Wu, Sens. Actuators, B, 2012, 166–167, 708–714 CrossRef CAS.
- Y. Song, K. Qu, C. Zhao, J. Ren and X. Qu, Adv. Mater., 2010, 22, 2206–2210 CrossRef CAS PubMed.
- Y. Song, X. Wang, C. Zhao, K. Qu, J. Ren and X. Qu, Chem.–Eur. J., 2010, 16, 3617–3621 CrossRef CAS PubMed.
- W. Shi, Q. Wang, Y. Long, Z. Cheng, S. Chen, H. Zheng and Y. Huang, Chem. Commun., 2011, 47, 6695–6697 RSC.
- L. Su, J. Feng, X. Zhou, C. Ren, H. Li and X. Chen, Anal. Chem., 2012, 84, 5753–5758 CrossRef CAS PubMed.
- K. Zhang, W. Zuo, Z. Wang, J. Liu, T. Li, B. Wang and Z. Yang, RSC Adv., 2015, 5, 10632–10640 RSC.
- T. Tian, L. Ai, X. Liu, L. Li, J. Li and J. Jiang, Ind. Eng. Chem. Res., 2015, 54, 1171–1178 CrossRef CAS.
- H. Wei and E. Wang, Chem. Soc. Rev., 2013, 42, 6060–6093 RSC.
- Y. Gu, Y. Xu and Y. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 801–806 CAS.
- Z. Dai, S. Liu, J. Bao and H. Ju, Chem.–Eur. J., 2009, 15, 4321–4326 CrossRef CAS PubMed.
- S. K. Maji, A. K. Dutta, S. Dutta, D. N. Srivastava, P. Paul, A. Mondal and B. Adhikary, Appl. Catal., B, 2012, 126, 265–274 CrossRef CAS.
- W. He, H. Jia, X. Li, Y. Lei, J. Li, H. Zhao, L. Mi, L. Zhang and Z. Zheng, Nanoscale, 2012, 4, 3501–3506 RSC.
- D. He, D. Wu, J. Gao, X. Wu, X. Zeng and W. Ding, J. Power Sources, 2015, 294, 643–649 CrossRef CAS.
- J. Li, X. Zhou, Z. Xia, Z. Zhang, J. Li, Y. Ma and Y. Qu, J. Mater. Chem. A, 2015, 3, 13066–13071 CAS.
- G. Wang and S. Zhuo, Phys. Chem. Chem. Phys., 2013, 15, 13801–13804 RSC.
- Q. Wang, L. Jiao, H. Du, W. Peng, Y. Han, D. Song, Y. Si, Y. Wang and H. Yuan, J. Mater. Chem., 2011, 21, 327–329 RSC.
- Y. Sun, C. Liu, D. C. Grauer, J. Yano, J. R. Long, P. Yang and C. J. Chang, J. Am. Chem. Soc., 2013, 135, 17699–17702 CrossRef CAS PubMed.
- S.-J. Bao, C. M. Li, C.-X. Guo and Y. Qiao, J. Power Sources, 2008, 180, 676–681 CrossRef CAS.
- P. Qu, Z. Gong, H. Cheng, W. Xiong, X. Wu, P. Pei, R. Zhao, Y. Zeng and Z. Zhu, RSC Adv., 2015, 5, 106661–106667 RSC.
- S. Xiong, J. S. Chen, X. W. Lou and H. C. Zeng, Adv. Funct. Mater., 2012, 22, 861–871 CrossRef CAS.
- B. You, N. Jiang, M. Sheng and Y. Sun, Chem. Commun., 2015, 51, 4252–4255 RSC.
- F. Bai, H. Huang, C. Hou and P. Zhang, New J. Chem., 2016, 40, 1679–1684 RSC.
- G. Valiulienė, A. Žielienė and J. Vinkevičius, J. Solid State Electrochem., 2002, 6, 396–402 CrossRef.
- C.-Y. Lin, D. Mersch, D. A. Jefferson and E. Reisner, Chem. Sci., 2014, 5, 4906–4913 RSC.
- Y. Sun, L. Yang, M. Wei, G. Chen, G. Che, J. Yang, Z. Wang and Z. Gao, J. Mater. Sci.: Mater. Electron., 2016, 27, 1457–1462 CrossRef CAS.
- Y. Su, Y. Zhang, X. Zhuang, S. Li, D. Wu, F. Zhang and X. Feng, Carbon, 2013, 62, 296–301 CrossRef CAS.
- J.-Y. Lin and S.-W. Chou, RSC Adv., 2013, 3, 2043–2048 RSC.
- G. V. Buxton, C. L. Greenstock, W. P. Heiman and A. B. Ross, J. Phys. Chem. Ref. Data, 1988, 17, 513–886 CrossRef CAS.
- L. Guo, Y. Xu, A. R. Ferhan, G. Chen and D.-H. Kim, J. Am. Chem. Soc., 2013, 135, 12338–12345 CrossRef CAS PubMed.
- Z. Mohammadpour, A. Safavi and M. Shamsipur, Chem. Eng. J., 2014, 255, 1–7 CrossRef CAS.
- X. Liu, Q. Wang, Y. Zhang, L. Zhang, Y. Su and Y. Lv, New J. Chem., 2013, 37, 2174–2178 RSC.
- M. Ravichandran, Chemosphere, 2004, 55, 319–331 CrossRef CAS PubMed.
- H.-C. Chang, Y.-F. Chang, N.-C. Fan and J. A. Ho, ACS Appl. Mater. Interfaces, 2014, 6, 18824–18831 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra16619a |
| This journal is © The Royal Society of Chemistry 2016 |