Combination of homogenous liquid–liquid extraction and dispersive liquid–liquid microextraction for extraction and preconcentration of amantadine from biological samples followed by its indirect determination by flame atomic absorption spectrometry
Abstract
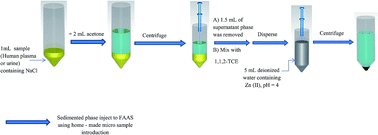
A new and simple procedure has been developed for the indirect determination of amantadine in biological samples. In this paper, a homogenous liquid–liquid extraction method followed by a dispersive liquid–liquid microextraction method has been developed for the extraction and preconcentration of amantadine from samples and its determination was performed by flame atomic absorption spectrometry. Initially, to human plasma or urine, sodium chloride and acetone were added. After manual shaking, the mixture was centrifuged and a two-phase system was formed: an upper phase of acetone containing amantadine and a lower phase containing soluble compounds in water and the precipitated proteins. The upper phase was removed, mixed with 1,1,2-trichloroethane as an extraction solvent at a microliter level and rapidly injected by a syringe into an aqueous solution containing Zn(II) with a pH of 4 placed into a test tube with a conical bottom. In this process, an amantadine–Zn complex was formed and extracted into fine droplets of the extraction solvent. After centrifugation, the fine droplets of the extractant containing the complex were sedimented in the bottom of the tube. The sedimented phase was injected to the detection system via a home-made sample introduction system. Under the optimal conditions, the linear ranges were between 3–400 ng mL−1. The limits of detection of the target analyte were obtained as 1.8 and 1.1 ng mL−1 in human plasma and urine, respectively.

 Please wait while we load your content...
Please wait while we load your content...