Acclimated sediment microbial fuel cells from a eutrophic lake for the in situ denitrification process
Yuan Wanga,
Jiangjun Hua,
Longmian Wangb,
Dan Shanc,
Xu Wang*a,
Yimin Zhangb,
Xuhui Maoa,
Lei Xingd and
Dihua Wanga
aSchool of Resource and Environmental Sciences, Wuhan University, No. 129 Luoyu Road, Wuhan 430079, China. E-mail: xu.wang@whu.edu.cn; Tel: +86-27-68775637
bNanjing Institute of Environmental Sciences, Ministry of Environmental Protection, No. 8 Jiang Wang Miao Street, Nanjing 210042, China
cSino-Japan Friendship Center for Environmental Protection, No. 1 Yu Hui Nan Road, Beijing 100029, China
dInstitute of Green Chemistry and Chemical Technology, Jiangsu University, No. 301 Xuefu Road, Zhenjiang, 212013, China
First published on 11th August 2016
Abstract
Sediment microbial fuel cells (SMFCs) with various external resistances were acclimated at the north of Lake Taihu for two months. Then, each SMFC was transferred to a container and operated in batch mode for evaluating its electrochemical and denitrification performances. The nitrate removal efficiencies of SMFCs that were connected to the external resistances of 510, 100, 51, 10 and 1 Ω were 12%, 20%, 32%, 42.1% and 60%, respectively. Conversely, the nitrate concentration increased by 0.05 mg L−1 for the SMFC with 1000 Ω external resistance. The microbial community of the biocathode in the SMFC with 1 Ω external resistance was determined. Proteobacteria (48.04%), Bacteroidetes (9.87%) and Chloroflexi (9.8%) were identified as the dominant phyla. The microbial community analysis and half-cell tests revealed that the acclimated biocathode could simultaneously promote a denitrification reaction and an oxygen reduction reaction. In addition, Nitrosomonadales and Nitrospirales on the biocathode facilitated the ammonia oxidation reaction which caused a mixed potential. The mixed potential was enhanced by increasing ammonia concentration and decreased the biocathode performance.
1. Introduction
Nitrate pollution from the effluent discharge of wastewater treatment plants and agricultural runoffs has presented serious environmental concerns such as eutrophication and human health threats.1,2 For example, the nitrogen loading into Lake Taihu in China has surpassed the environmental capability and caused severe nitrogen pollution, especially in the form of nitrate.2,3 Lake Taihu acts as the main drinking water source for surrounding cities and so the nitrogen pollution causes problems. When the water containing nitrate is ingested by infants, it can cause methemoglobinemia (blue baby syndrome).4 Meanwhile, nitrate may also participate in the formation of carcinogenic nitrosamine compounds.5,6 In addition, in laboratory animal tests, it has been confirmed that nitrate can cause heart and behavioral problems.6Ion exchange, reverse osmosis and electrodialysis have been investigated for use in nitrate removal.7,8 However, these methods are energy intensive and may cause secondary pollution if the brine waste is not handled properly and so biological denitrification has been considered as a low cost and effective alternative.9 In this process, heterotrophic denitrifiers utilize (added) organic substrates that act as electron donors, and convert nitrate into nitrogen under anoxic conditions. The drawback of this process is that the nitrate cannot be removed effectively if the supply of carbon substrate does not meet the stoichiometric requirement. However, the excessive supply of carbon substrates will result in secondary pollution [chemical oxygen demand (COD)]. Therefore, a cost-effective, in situ application and environmentally friendly denitrification method is highly desired for nitrate removal from natural water bodies.10
Sediment microbial fuel cells (SMFCs) were initially investigated for powering remote sensors. Recently, their effectiveness for power generation as well as environmental remediation has been demonstrated.11–13 Unlike heterotrophic denitrifiers, SMFCs utilize the organic matter in the sediment as a carbon substrate to reduce nitrate and therefore secondary pollution is avoided. Zhang and Angelidaki reported nitrate and nitrite reduction for eutrophic lakes using the biocathode of SMFCs.13 However, these SMFCs were acclimated in favorable conditions (e.g., deoxygenated solution, use of high concentration nitrate salt and so on) for incubating the denitrification bacteria on the cathode. The real lake environment is far different from the conditions described previously, it is more complex (e.g., oxygen, ammonia nitrogen and other pollutants are present) and the concentration of nutrients is much lower. For the in situ denitrification, SMFCs need to stay in the real lake environment for a long time. The bacterial community of the biocathode that was acclimated from controlled conditions will be significantly changed. It is uncertain whether the biocathode of SMFCs could still facilitate the denitrification reaction after the long-term operation in a real lake environment.
SMFCs with various external resistances were acclimated in the north of Lake Taihu for two months. It is believed that this is the first time that a biocathode obtained from the real lake environment was examined in terms of microbial community, and electrochemical and denitrification performance. The impact of dissolved oxygen, ammonia nitrogen and nitrate in the lake water on biocathode performance was also investigated. This study verified the feasibility of in situ denitrification via the biocathode of SMFCs in the real lake environment.
2. Methods
2.1 SMFC setup and operation
The field experiment was conducted at the north of Lake Taihu (31°40′12.96′′N, 119°47′46.16′′E) (as shown in Fig. 1a). According to the Standard Methods for the Examination of Water and Wastewater,14 the lake water was analyzed to determine permanganate index (CODMn; 6.0 mg L−1), dissolved oxygen (DO, 2.3 mg L−1), total nitrogen (4.1 mg L−1), nitrate–nitrogen (NO3−–N; 2.5 mg L−1), nitrite–nitrogen (NO2−–N; 0.08 mg L−1), ammonium–nitrogen (NH4+–N; 1.3 mg L−1), total phosphorus (0.3 mg L−1), pH (7.6) and conductivity (450 μS cm−1). Considering the very low concentration of NO2−–N, it was excluded for any further tests. The sediment thickness was 8.0 cm and the organic matter in sediment was measured using the loss on ignition (LOI) technique reported by Hong et al.15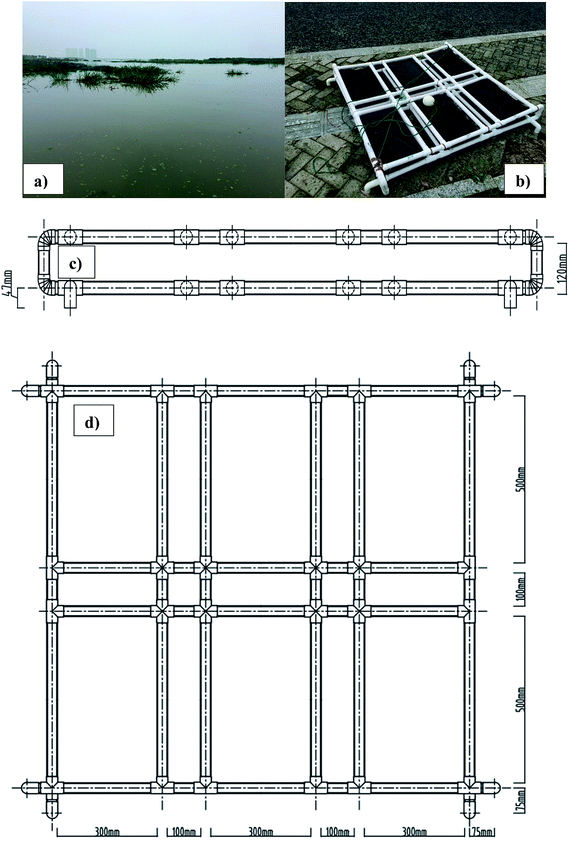 | ||
| Fig. 1 (a) Site view of field experiment, (b) SMFCs attached on the frame, (c) front view of frame design and (d) top view of frame design. | ||
SMFCs were operated for two months (from August to October, 2015) under six different external resistances (1000 Ω, 510 Ω, 100 Ω, 51 Ω, 10 Ω and 1.0 Ω). The electrodes of the SMFCs were made of graphite felt (Zhongxin Co. Ltd, China) with a length of 30 cm, a width of 50 cm and a thickness of 0.5 cm. They were washed thoroughly with ethanol and deionized water to eliminate the surface contamination. Then, the electrodes were fixed to a poly(vinyl chloride) pipe (30 mm in diameter) frame (as shown in Fig. 1c and d) to maintain the distance of the anodes and cathodes at 12 cm. The electrodes and external resistances were connected by titanium wire. Finally, epoxy resin was applied thoroughly onto the external resistances to protect them from corrosion.
2.2 Electrochemical characterization
After two months of operation, each SMFC was moved to a container (61 cm × 42 cm × 30 cm) for batch operation. Surface sediment samples (0–10 cm) were taken using a grab sampler (2 L) from the location where the field experiment was conducted. The container was filled with 25 L of lake water and the SMFC anode was buried in the sediment. No buffer, vitamins or minerals were added to the contents of the container and it was left open to the atmosphere to simulate the conditions of the natural environment.The voltage of the SMFCs was automatically measured at an interval of 10 min using a model 2700 data acquisition system (Keithley Instruments, USA). Polarization curves were obtained by varying the external resistance from 1000, 510, 100, 51, 10 to 1 Ω.
The half-cell test was conducted in a 250 mL glass bottle using the three electrode system which consisted of a working electrode (biocathode from 1 Ω SMFC), a silver/solver chloride (Ag/AgCl) reference electrode and a platinum coil counter electrode. Linear sweep voltammetry (LSV) was recorded using a CHI 660E potentiostat (CHI Instruments) with a scan rate of 1 mV s−1. No buffer, vitamins or minerals were added to the contents of the bottle. Helium gas and standard gases containing either 100% or 21% of oxygen were used to control the DO level in the glass bottle. The concentration of NO3−–N and ammoniacal–nitrogen (NH3–N) was adjusted by adding sodium nitrate or ammonia chloride, respectively.
2.3 Microbial community analysis
The water sample from Lake Taihu was firstly filtered with a 0.22 μm membrane. For high throughput MiSeq sequencing (Illumina) of the 16S rDNA, biofilms on the graphite felt cathode of the 1 Ω SMFC were sampled after two months by repeatedly scraping the cross section using a sterile blade. The DNA was extracted from 250 mg sample using the MoBio PowerSoil DNA isolation kit (MO BIO Laboratories, Carlsbad, CA, USA) following the manufacturer's instructions. The V4–V5 region of the bacterial 16S rRNA genes were amplified using the universal primers 515F (GTGCCAGCMGCCGCGGTAA) and 926R (CCGTCAATTCMTTTRAGTTT) because of their good coverage of almost all phyla in conventional and metagenomic studies.16–18 In addition, the primers also contained the Illumina 5′ overhang adapter sequences for building the two-step amplicon library according to the manufacturer's instructions.The initial polymerase chain sequencing (PCR) reactions were carried out in a 25 μL reaction volume with 1 μL of DNA template, 250 μM of deoxynucleotides (dNTP), 0.25 μM of 515F primer, 0.25 μM 926R primer, 1X reaction buffer, and 0.5 U Phusion DNA Polymerase (New England Biolabs, MA, USA). The PCR conditions were: initial denaturation at 94 °C for 2 min, 25 cycles of denaturation at 94 °C for 30 s, annealing at 56 °C for 30 s and extension at 72 °C for 30 s, and a final extension of 72 °C for 5 min. The Nextera XT Index kit (Illumina, San Diego, CA, USA) with dual 8-base barcodes were used for multiplexing. Eight cycles of PCR reactions were used to incorporate two unique barcodes to either end of the 16S amplicons. Cycling conditions consisted of one cycle of 94 °C for 3 min, eight cycles of 94 °C for 30 s, one cycle of 56 °C for 30 s, one cycle of 72 °C for 30 s, and a final extension cycle of 72 °C for 5 min. Prior to library pooling, the barcoded PCR products were purified using an AxyPrep DNA gel extraction kit (Axygen, China) and quantified using the Qubit dsDNA HS Assay Kit (Life Technologies, Carlsbad, CA, USA). The libraries were sequenced using 2 × 300 bp paired-end sequencing on the MiSeq platform using a MiSeq v3 Reagent Kit (Illumina). All the sequences were sorted into different samples according to the barcodes. Related data were returned as a FASTQ file of forward or backward read sequences for each sample.
Sequence preprocessing was performed mainly using mothur software (version 1.35.1),19 following the MiSeq analysis pipeline outlined at http://www.mothur.org/wiki/MiSeq_SOP. Contigs were assembled from paired-end reads into a single FASTA file of complete sequences. The sequences were filtered to remove ambiguous reads shorter than 200 bp or longer than 600 bp. The rest sequences were simplified using the ‘unique.seqs’ command to generate a unique set of sequences, and then aligned with the SILVA database, version 119.20 Here, the chimeric sequences were also removed using the ‘chimera.uchime’ command with default parameters. The candidate sequences were assigned to the taxonomy using the ‘classify.seqs’ command (Wang approach) with a confidence threshold of 80%. Any sequences aligned to eukaryota, chloroplasts or mitochondria, as well as unknown sequences, were discarded. Then, the distance matrix between the aligned sequences was generated by the ‘dist.seqs’ command. Finally, these sequences were clustered to operational taxonomic units (OTUs) at 97% sequence identity (the furthest neighbor method). The majority consensus taxonomy for each OTU was obtained by the ‘classify.otu’ command with default parameters. OTU taxonomies of phyla, class and order were based on the National Center for Biotechnology (NCBI) database. The number of sequences at different classification level was summarized using the self-compiled script. Abundance of the species at all levels was calculated using each species of sequence number divided by the total sequence number of the sample.
3. Results and discussion
3.1 Electrochemical performance of SMFCs under different external resistances
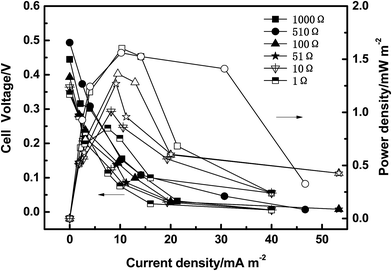
Fig. 2 shows the polarization curves and power densities of SMFCs under six different external resistances (1000, 510, 100, 51, 10 and 1 Ω) after two months operation. The open circuit potential (OCP) of SMFCs that were acclimated under various external resistances increased from 0.34 V to 0.49 V when a higher external resistance was used. The power density was derived from the polarization curves, which specify the performance differences of the SMFCs. This follows an order of 1000 Ω (1.6 mW m−2) > 510 Ω (1.56 mW m−2) > 100 Ω (1.36 m Wm−2) > 51 Ω (1.27 mW m−2) > 10 Ω (1.0 mW m−2) > 1 Ω (0.85 mW m−2). Song et al. reported that the electrode potential (including OCP) and power output were improved by connecting SMFCs to higher external resistances (up to 1000 Ω) during acclimatization.21 In this study, the highest power density was 1.6 mW m−2, which was lower than the previously reported values in the literature.15,22 This is mainly because of the low nitrate concentration (2.5 mg L−1) and DO (2.3 mg L−1) of the lake water in this study, compared to the synthetic wastewater that used in reports in the literature.13,153.2 Nitrate removal performance
To investigate the nitrate removal performance, SMFCs were operated in batch mode, and the voltage output of SMFCs was monitored for around 115 h. In this study, SMFCs were investigated for nitrate removal from the eutrophic lake rather than for powering a remote sensor, so the current output of SMFCs plays a more important role than the power density. After converting voltage output to current output, it can be seen in Fig. 3a that the current output of SMFCs is higher when smaller external resistance was used. In Fig. 3b, the corresponding nitrate concentration in the containers with the external resistances of 1000 Ω, 510 Ω and 100 Ω decreases at first, and then starts to rise after around 50 h. In Lake Taihu, the nitrification process can be promoted because of the presence of ammonia-oxidizing bacteria and archaea.23 During the initial 50 h, nitrate could be removed efficiently through a possibly heterotrophic denitrification process using the COD that was absorbed on the electrode material as the carbon substrate. After 50 h, the COD on the electrode was depleted and heterotrophic denitrification occurred inefficiently. In addition, the current output of the SMFCs with high external resistance (1000, 510 and 100 Ω) was small which means that the electrochemical denitrification process was relatively slow. Therefore, nitrate concentration in these SMFC reactors started to increase after around 50 h. The SMFCs with smaller external resistances, however, could sustain the nitrate removal through the electrochemical denitrification process. The nitrate removal efficiencies of the SMFCs with the external resistances of 510 Ω, 100 Ω, 51 Ω, 10 Ω and 1 Ω were 12%, 20%, 32%, 42.1% and 60%, respectively. A previous report by Zhang and Angelidaki also suggested that high nitrate removal efficiency could be achieved by using a low external resistance or even a close circuit.13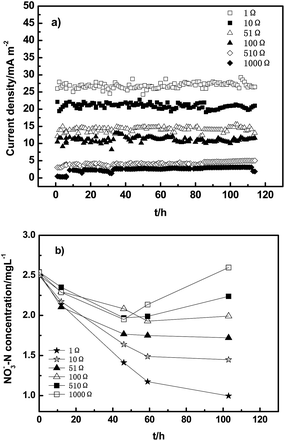 | ||
| Fig. 3 (a) Current output of SMFCs and (b) variations of nitrate concentration during the batch mode test. | ||
3.3 Microbial community analysis of the SMFC biocathode
To identify the microbial community in the biocathode of the SMFC with 1 Ω external resistance and the lake water, the qualified reads were assigned to known phyla, classes and orders. Fig. 4a shows that Proteobacteria was the most abundant phylum in both the biocathode and lake water, with a relative abundance of 48.04% and 47.62%, respectively. Other dominant phyla in the biocathode were Bacteroidetes (9.87%), Chloroflexi (9.8%), and in the lake water, Actinobacteria (20.79%), Bacteroidetes (10.37%). The microbial community of the biocathode in denitrification microbial fuel cells (MFCs) from previous publications shared the same predominant phylum of Proteobacteria, followed by Firmicutes and Chloroflexi.24 It has been reported that the microbial community of the biocathode in an aqueous air cathode MFC was comprised mainly of Proteobacteria, Planctomycetes and Bacteroidetes.25 Because of the presence of DO and nitrate in the lake water, the biocathode in the present study contained the dominant phyla from both the denitrifying cathode and the air cathode. | ||
| Fig. 4 Microbial community analysis of (a) phyla distribution on the SMFC cathode and lake water, and (b) ten dominant orders on the SMFC cathode. | ||
Fig. 4b shows the ten most abundant bacterial orders found on the SMFC biocathode. The orders of Burkholderiales and Rhodocyclales from the β-Proteobacteria class were identified as being dominant in denitrifying cathodes.24,26 Nitrosomonadales and Nitrospirales are known as ammonia oxidizing bacteria and Nitrosomonadales could also function in a denitrifying manner in a loop system.24,27 Ammonia oxidizing bacteria on the biocathode in this study were incubated because of the presence of NH3–N and DO in the lake water. Anaerolineales, which belong to the Chloroflexi, was the most abundant order and they were identified as dominant in the denitrifying cathodes in a previous study.24 In addition, Pseudomonadales from γ-Proteobacteria and Sphingobacteriales from Bacteroidetes were found to have the ability of catalyzing an oxygen reduction reaction.28–30
3.4 Effect of NO3−–N, NH3–N or DO concentration on the cathode performance
The microbial community analysis described in the previous section suggested that the denitrification, oxygen reduction and ammonia oxidation bacteria were the dominant orders on the SMFC cathode. Thus, it is essential to investigate the impact of NO3−–N, NH3–N or DO concentration on the performance of the 1 Ω SMFC biocathode. Fig. 5 shows that the concentration of NO3−–N, NH3–N or DO has a significant effect on the biocathode performance. The biocathode performance presents an enhancing trend with the increase of oxygen or nitrate concentration (as shown in Fig. 5a and b). Virdis et al. found that the denitrification process was inhibited when the DO level exceeded 4.35 mg L−1 in a two chamber MFC.31 Zhang and Angelidaki also observed a similar phenomenon in the SMFCs.13 In Fig. 5c, the biocathode performance decreases with increasing NH3–N concentration. In direct alcohol fuel cells, anode fuel could pass through the ion exchange membrane and result in a performance drop because of the mixed potential caused by oxidation and reduction reactions that take place simultaneously at the cathode.32 In this study, the mixed potential mainly originated from the NH3–N oxidation reaction and the reduction reactions (DO and NO3−–N). | ||
| Fig. 5 LSVs of the SMFC biocathode under different (a) NO3−–N concentrations, (b) DO concentrations and (c) NH3–N concentrations. | ||
4. Conclusions
The in situ denitrification was demonstrated by using the biocathodes of SMFCs that were acclimated in a eutrophic lake. The SMFC with a small external resistance was more effective for the in situ denitrification. The highest nitrate removal efficiency of 60% was achieved using the SMFC with a 1 Ω external resistance after 115 h operation in the batch mode operation. The acclimated SMFC biocathode contained mixed microbial communities which could facilitate denitrification, oxygen reduction and ammonia oxidation reactions. The presence of the elevated NH3–N concentration in the lake water caused a mixed potential at the biocathode of SMFC and led to a performance drop. Future research will focus on optimizing the electrode design and improving the reaction selectivity of biocathode for the nitrate reduction reaction.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Grant No. 21406171 and No. 21173184), the National Science and Technology Support Project (Grant No. 2012BAJ21B00), the Natural and Science Foundation of Hubei Province (Grant No. ZRY2014001946), the Fundamental Research Funds for the Central Universities (Grant No. 2042014kf0046) and the foundation research project of Jiangsu Province (Grant No. BK20161100).References
- B. C. Kross, G. R. Hallberg, D. R. Bruner, K. Cherryholmes and J. K. Johnson, Am. J. Public Health, 1993, 83, 270–272 CrossRef CAS PubMed.
- L. Shan, Y. He, J. Chen, Q. Huang, X. Lian, H. Wang and Y. Liu, Agr. Water Manag., 2015, 159, 255–263 CrossRef.
- Y. Zhao, Y. Xia, C. Ti, J. Shan, B. Li, L. Xia and X. Yan, Environ. Sci. Technol., 2015, 49, 1427–1435 CrossRef CAS PubMed.
- H. I. Shuval and N. Gruener, in Proceedings of the Conference on Nitrogen As a Water Pollutant, Pergamon, 2013, pp. 183–193, DOI:10.1016/B978-1-4832-1344-6.50017-4.
- S. S. Mirvish, in Proceedings of the Conference on Nitrogen As a Water Pollutant, Pergamon, 2013, pp. 195–207, DOI:10.1016/B978-1-4832-1344-6.50018-6.
- N. S. Bryan, D. D. Alexander, J. R. Coughlin, A. L. Milkowski and P. Boffetta, Food Chem. Toxicol., 2012, 50, 3646–3665 CrossRef CAS PubMed.
- J. P. van der Hoek, P. J. M. van der Ven and A. Klapwijk, Water Res., 1988, 22, 679–684 CrossRef CAS.
- H. I. Park, D. k. Kim, Y.-J. Choi and D. Pak, Process Biochem., 2005, 40, 3383–3388 CrossRef CAS.
- R. Li, C. Feng, W. Hu, B. Xi, N. Chen, B. Zhao, Y. Liu, C. Hao and J. Pu, Water Res., 2016, 89, 171–179 CrossRef CAS PubMed.
- Q. Yang, H. Zhao and H. Liang, Bioresour. Technol., 2015, 197, 512–514 CrossRef CAS PubMed.
- C. E. Reimers, L. M. Tender, S. Fertig and W. Wang, Environ. Sci. Technol., 2001, 35, 192–195 CrossRef CAS PubMed.
- S. W. Hong, J. Kim, S. C. Yong and H. C. Tai, Bull. Korean Chem. Soc., 2008, 29, 2189–2194 CrossRef CAS.
- Y. Zhang and I. Angelidaki, Water Res., 2012, 46, 6445–6453 CrossRef CAS PubMed.
- American Public Health Association (APHA), Standard Methods for the Examination of Water and Wastewater, APHA-AWWA-WEF, Washington, DC, 22nd edn, 2012 Search PubMed.
- S. W. Hong, I. S. Chang, Y. S. Choi, B. H. Kim and T. H. Chung, Bioprocess Biosyst. Eng., 2008, 32, 389–395 CrossRef PubMed.
- G. C. Baker, J. J. Smith and D. A. Cowan, J. Microbiol. Methods, 2003, 55, 541–555 CrossRef CAS PubMed.
- Z. Liu, T. Z. DeSantis, G. L. Andersen and R. Knight, Nucleic Acids Res., 2008, 36, e120 CrossRef PubMed.
- Y. Wang and P. Y. Qian, PLoS One, 2009, 4, e7401 Search PubMed.
- P. D. Schloss, S. L. Westcott, T. Ryabin, J. R. Hall, M. Hartmann, E. B. Hollister, R. A. Lesniewski, B. B. Oakley, D. H. Parks, C. J. Robinson, J. W. Sahl, B. Stres, G. G. Thallinger, D. J. Van Horn and C. F. Weber, Appl. Environ. Microbiol., 2009, 75, 7537–7541 CrossRef CAS PubMed.
- E. Pruesse, C. Quast, K. Knittel, B. M. Fuchs, W. Ludwig, J. Peplies and F. O. Glockner, Nucleic Acids Res., 2007, 35, 7188–7196 CrossRef CAS PubMed.
- T.-S. Song, Z.-S. Yan, Z.-W. Zhao and H.-L. Jiang, J. Chem. Technol. Biotechnol., 2010, 85, 1489–1493 CAS.
- J. Zhao, X.-F. Li, Y.-P. Ren, X.-H. Wang and C. Jian, J. Chem. Technol. Biotechnol., 2012, 87, 1567–1573 CrossRef CAS.
- J. Hou, C. Song, X. Cao and Y. Zhou, Water Res., 2013, 47, 2285–2296 CrossRef CAS PubMed.
- K. C. Wrighton, B. Virdis, P. Clauwaert, S. T. Read, R. A. Daly, N. Boon, Y. Piceno, G. L. Andersen, J. D. Coates and K. Rabaey, ISME J., 2010, 4, 1443–1455 CrossRef CAS PubMed.
- Z. Wang, Y. Zheng, Y. Xiao, S. Wu, Y. Wu, Z. Yang and F. Zhao, Bioresour. Technol., 2013, 144, 74–79 CrossRef CAS PubMed.
- H. I. Park, J. S. Kim, D. K. Kim, Y.-J. Choi and D. Pak, Enzyme Microb. Technol., 2006, 39, 453–458 CrossRef CAS.
- H. Daims, E. V. Lebedeva, P. Pjevac, P. Han, C. Herbold, M. Albertsen, N. Jehmlich, M. Palatinszky, J. Vierheilig, A. Bulaev, R. H. Kirkegaard, M. von Bergen, T. Rattei, B. Bendinger, P. H. Nielsen and M. Wagner, Nature, 2015, 528, 504–509 CAS.
- A. Cournet, M.-L. Délia, A. Bergel, C. Roques and M. Bergé, Electrochem. Commun., 2010, 12, 505–508 CrossRef CAS.
- P. Clauwaert, D. van der Ha, N. Boon, K. Verbeken, M. Verhaege, K. Rabaey and W. Verstraete, Environ. Sci. Technol., 2007, 41, 7564–7569 CrossRef CAS PubMed.
- K. Rabaey, S. T. Read, P. Clauwaert, S. Freguia, P. L. Bond, L. L. Blackall and J. Keller, ISME J., 2008, 2, 519–527 CrossRef CAS PubMed.
- B. Virdis, K. Rabaey, R. A. Rozendal, Z. Yuan and J. Keller, Water Res., 2010, 44, 2970–2980 CrossRef CAS PubMed.
- E. H. Yu, X. Wang, U. Krewer, L. Li and K. Scott, Energy Environ. Sci., 2012, 5668–5680 CAS.
| This journal is © The Royal Society of Chemistry 2016 |