pH dependent size control, formation mechanism and antimicrobial functionality of bio-inspired AgNPs†
Venkatanarasimha Rao Chelli and
Animes Kumar Golder *
*
Department of Chemical Engineering, Indian Institute of Technology, Guwahati, Assam-781039, India. E-mail: animes@iitg.ernet.in; Fax: +91-361-2582292; Tel: +91-361-2582269
First published on 30th September 2016
Abstract
Sechium edule is rich in ascorbic acid which was extracted from aqueous media for the synthesis of AgNPs. This work reports the effects of pH on the kinetics and mechanisms of AgNPs formation using this extract leading to different particle sizes, morphology, spectral response, and antimicrobial functionality. Thermodynamically facile Ag2O reduction at a higher pH (≥9) resulted in spherical particles of smaller sizes; however, the particles were laden with a trace of Ag2O at pH 12.5. A broad and bimodal distribution of AgNPs of different shapes and sizes were originated at a lower pH (3 ≤ pH ≤ 5) from Ag+ and Ag2O reductions where ascorbic acid mostly exists as dehydro-ascorbic acid. A single surface plasmon resonance peak at 425 nm exhibited a blue shift with the decrease in AgNPs size and increase in sphericity in the abundance of OH− ions. The silver–ascorbate layer induced particles destabilization along with the formation of ascorbate free radical (g-factor: 2.0052 to 2.0056) which was also responsible for the inhibition of B. subtilis and E. coli.
1. Introduction
Nanomaterials are commonly used in sectors like energy generation, electronics, textiles, cosmetics, optics, medicinal, and environmental sciences.1,2 Nanoparticles of noble metals such as silver, platinum, and gold, in particular, have specific implications in biology and nanomedicine.3 Amongst these, silver nanoparticles (AgNPs) have unique properties such as strong surface plasmon resonance (SPR), excellent conductivity, catalytic activity, chemical stability, and significant antifungal, antibacterial and antiviral activities.4 The thermochemical techniques of nanoparticle fabrication are known for the use of harmful chemicals and energy intensive processes. Hydrazine is a common analyte in the field of nanoparticles synthesis5 but it is toxic and dangerously unstable.6 Aniline, a chemical of severe environmental concern, is used for the synthesis of modified silver nanocrystals of various morphologies.7,8 Ethanediol and hydroquinone are also employed for the nanoparticles synthesis.9,10 The UV irradiation technique involves a high operation cost, and so it is energy inefficient.11 It also can cause the health hazard to the working personnel.Thus, a greener route of environmentally benign nanoparticles synthesis has gained tremendous success in recent times. The bio-mediated methods are straightforward and eco-friendly and, the whole process can be carried out at room temperature and pressure.12 Intercellular synthesis of nanoparticles using microbes often shows a lower synthesis rate as well as difficulty in size and shape control.13 Such problems can be overcome in many extents by extracting the analyte in a suitable media from the living cells. After that, the particles are synthesized in this media. For examples, water soluble analytes such as ascorbic acid (AA)14 and polyphenols15 including flavonoids13 are extracted in water for AgNPs synthesis. However, the extraction pH could degrade such compounds.
Polyphenolic compounds present in plant extracts viz., caffeic, chlorogenic, and gallic acids are decomposed irreversibly when they are exposed to a high pH (>9).16 AA is converted to dehydro-AA at pH ≤ 4.2.17 The organs of the plants such as Ocimum sanctum leaf,18 Citrus limon,19 Citrus sinensis peel,20 tea leaf,21 and Manilkara zapota fruit22 laden with AA are used for AgNPs synthesis. However, the fate of AA with the change in pH and its effect on the quality and purity of particles synthesized using a bio-mediated process is still not known. On the other hand, higher pH increases the stability of colloid formation and cluster distribution due to repulsive electrosteric interactions.2 It also enhances the nucleation and growth rates with the subsequent crystallization leading to the formation of smaller particles.23,24
Therefore, the focus of this work is a pH-dependent synthesis of AgNPs in the aqueous extract of Sechium (S.) edule which is rich in AA. It primarily covers the effect of pH on the formation kinetics of AgNPs, particles purity, and particle sizes along with the fates of analytes and influences of Ag2O which is readily formed at a higher pH. The antibacterial activity of AgNPs is also tested following a typical protocol against E. coli and B. subtilis.
2. Materials and methods
2.1. Reagents
AgNO3 (99%), ethanol (98%), H2SO4 (99%), and NaOH (99%) were purchased from Merck, Mumbai, India. Streptomycin (S-10, IP 200 mcg per disc) discs, ampicillin (Amp, IP 200 mcg per disc) discs, tryptone (CR014-500G), agar (CAS no. 9002-18-0), and yeast extract (34266RM027) were procured from Himedia, Mumbai, India. Escherichia coli and Bacillus subtilis were supplied by National Chemical Laboratory, Pune, India. Deionized water (dH2O) of Millipore water purification unit (Elix-3, California, USA) was used to prepare all the reagents and bio-extract.2.2. Preparation of bio-extract
S. edule was purchased from the market located at Indian Institute of Technology Guwahati, India. The cleaned fruits were cut into small pieces and, the bio-extract was prepared by heating 1 g fruit pieces per 8 mL dH2O for 12 h at 90 ± 2 °C. The clear bio-extract was collected after the filtration using the nylon mesh followed by the membrane filter (0.45 μm, Pall membrane filter, India). The color of the bio-extract was slightly yellowish (Fig. 1) without a distinct spectral absorption peak but, it exhibited a broad absorption band between 200 and 250 nm (Fig. S1 of ESI†). The fresh bio-extract was employed immediately for the AgNPs synthesis. | ||
| Fig. 1 Spectral absorbance of bio-extract and AgNO3 mixture at different pH. Reaction time 24 h and room temperature (25 ± 2 °C). Inset: color variation at the end of 24 h reaction. | ||
2.3. AgNPs synthesis procedure
An equal volume of the bio-extract (pH 5.8) and AgNO3 (1 mM) solution was mixed with continuous agitation on a magnetic stirrer at 320 rpm (Spinot, Tarsons, India) in dark. The mixture pH was brought between 1 and 12.5 using 0.1 M H2SO4 or NaOH at the beginning of the reaction and, the reaction period was kept to be 24 h. The intermediate sample volume of 5 mL was collected and then centrifuged at 4000 rpm for 20 min (PR23, Remi instruments Ltd., Mumbai, India). The supernatant was analyzed for the residual Ag+ ions concentration and pH. The precipitate was washed with dH2O followed by ethanol for the removal of bio-residuals and, dried at 90 ± 5 °C in a hot-air oven for 12 h (N-101, Navyug, Kolkata, India). The dry powder was ground in a mortar and pestle for the subsequent analyses. The particles after the ethanol wash were re-dispersed by sonication for 20 min (UC-02, Jeio Tech, Daejeon, Korea) in dH2O for the determination of hydrodynamic diameter using the dynamic light scattering (DLS) technique.2.4. Characterizations and analytical techniques
Ag+ concentration was determined using atomic absorption spectroscopy (AA240FS, Varian, California, USA). pH was monitored using a pH meter (LI164, Elico, Hyderabad, India). The particles size and zeta-potential measurements were performed using Delsa nanoparticle analyzer (Delsa Nano-C, Beckman Coulter, Nyon, Switzerland). Transmission electron microscopic (TEM) analyses were carried out to obtain the micrograph, particles size, and selected area electron diffraction (SAED) pattern (JEM 2100, JEOL, Tokyo, Japan). 0.2 g AgNPs was added into 40 mL ethanol and ultrasonicated for 20 min to break the particles aggregation. A small drop of this suspension was soaked on the TEM grid and dried it for 1 h at 60 °C before the analysis. The same sample was also used for field emission scanning electron microscopy (FESEM) using a glass slide instead of a copper grid (Sigma, Zeiss, Jena, Germany). Thermogravimetric analysis (TGA) was carried out up to 950 °C with an N2 flow rate of 20 mL min−1 and a heating rate of 10 °C min−1 (TG 209 F1 Libra, Netzsch, Selb, Germany). Fourier transform infrared (FTIR) spectra were captured between 4000 and 500 cm−1 wavenumbers. X-ray diffractogram (XRD) was recorded with a scan rate of 5° per min using CuKα radiation (D8 Advance, Bruker, Karlsruhe, Germany). The mass spectrum was recorded by using a LC-TOF-MS (Waters Q-Tof Premier, USA) employing YMC Hydrosphere C18 reverse phase column. Electron spin resonance (ESR) spectra of AgNPs were obtained with a power and microwave frequency of 1 mW and 9.42 GHz (JES-FA200, JEOL, MA, USA).AA concentration in the bio-extract was measured by high performance liquid chromatography (26462, Shimadzu, Japan) using a C18 column (150 mm length, ∅ 3.5 mm) with a mobile phase consisting of acetic acid (0.05%) and acetonitrile (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 v/v) at a wavelength of 254 nm and flow rate of 1 mL min−1.
20 v/v) at a wavelength of 254 nm and flow rate of 1 mL min−1.
2.5. Antibacterial activity test for AgNPs synthesized at different pH
The growth kinetics of a Gram-positive bacterium, B. subtilis and, a Gram-negative bacterium, E. coli, were studied at various concentrations of AgNPs (2–50 μg mL−1).24 The test included a control consisting of a nutrient media and bacterial strains without AgNPs and with AgNPs. The Luria–Bertani medium was prepared by mixing 10 g L−1 NaCl, 10 g L−1 tryptone, and 5 g L−1 yeast extract. 20 mL of this media was taken in a glass tube and sterilized by autoclaving (NSW-229, Narang Scientific Works Pvt. Ltd., New Delhi, India) at 120 °C and 15 kg cm−2 pressure for 20 min. The test samples were incubated at 37 °C with an agitation speed of 160 rpm. All the tests were conducted in triplicate. The growth kinetics were evaluated by recording the change in the optical absorbance at 600 nm.253. Results and discussion
3.1. pH dependent AgNPs formation and effect of reaction time
AgNPs was synthesized by varying the pH from 3 to 12.5 and, the particles formed at pH 3 are designated as ‘AgNPs-pH 3’. Similar notation is used for AgNPs formed at different pH. The variations in the optical absorbance and color of the reaction mixture at different pH are shown in Fig. 1. There was no AgNPs formation at pH 1. So, no absorbance peak was identified. However, the absorption peak was found to appear with the further increase in pH. There was a minor peak at 428 nm at pH 3. The peak intensity was gradually enhanced and, shifted to a lower wavelength, i.e., to 427, 424, 417, 416, and 414 nm at pH 5, 7, 9, 11, and 12.5, respectively. These are the characteristic peaks of AgNPs in the suspension form resulted from the SPR effect26 which is mostly size and shape dependent. This hypsochromic shift is indicative of the reduction in the size and degree of anisotropy of the particles.27,28 The full width at half maximum decreased from 168.7 to 108.9 nm for AgNPs-pH 3 to AgNPs-pH 12.5 (Table S1 of the ESI†). It indicates the reduction in the polydispersity of the particles25 at a higher pH due to smaller particles size.The above results are also in line with the variation in solution color during synthesis (Fig. 1 (inset)). There was little change in the solution color even after 24 h of reaction with AgNPs-pH 3. The color was light red-brown in the case of AgNPs-pH 5 and, a dark red-brown color appeared with the particles formed at an elevated pH. The rate of Ag+ conversion to AgNPs was faster at the initial period with a higher rate at an elevated pH (Fig. 2). There was about 11.5% residual Ag+, i.e., 88.5% AgNPs-pH 12.5 was formed by 12 h and, it increased to 96% in 24 h. The yield of AgNPs in 24 h of the reaction was found to be 62.3, 77.4, 86.4, 93.4, and 94.7% at pH 3, 5, 7, 9, and 11, respectively.
The particles size distribution is shown in Fig. 3. The distribution width was reduced with the increase in pH. It was also shifted towards the lower particles size. So, the average particles size was reduced at a higher pH. The lowest and highest particles in differential number distribution varied from 51 to 193.9, 46.7 to 188.9, 34.4 to 130.9, 14.1 to 57.6, 11.8 to 44.4, and 7.1 to 10.9 nm for AgNPs-pH 3 to AgNPs-pH 12.5, respectively. The average hydrodynamic diameter followed a decreasing trend as 68.2, 60.1, 44.9, 31.2, 14.5, and 8.9 nm in which 90% AgNPs came under 87.6, 76.8, 57.6, 44.6, 17.8, and 9.2 nm, respectively.
The size, shape, and morphology of AgNPs-pH 3 and AgNPs-pH 9 were also examined using TEM (Fig. 4) and FESEM (Fig. S2 of ESI†). AgNPs-pH 3 size was higher with a significant agglomeration (Fig. 4(a)). However, the small particles were mostly separate and, the agglomeration was also reduced notably for AgNPs-pH 9 (Fig. 4(b)). The bright concentric circles (SAED patterns) depict the (111), (200), and (311) planes of the face-centered cubic (fcc) AgNPs crystals. The high-resolution TEM micrograph of a single AgNP showed the clear lattice fringes with an interplanar d-spacing of 0.2354 and 0.236 nm for AgNPs-pH 3 (Fig. 4(a)) and AgNPs-pH 9 (Fig. 4(b)), respectively. The mean, lowest, and highest particle size both in TEM and FESEM analyses were very close (Table S1 of the ESI†). However, the dry particles sizes of AgNPs-pH 3, AgNPs-pH 5.8,29 and AgNPs-pH 9 were about 80% lower than the particles size measurements in the aqueous media using DLS (Table S1 of the ESI†).
OH− ions preferably react with Ag+ yielding Ag2O particles (eqn (1)) (Fig. S3 of ESI†). Ag0 clusters would form on the surface of Ag2O. Eventually, it would grow to tiny AgNPs.1,30 However, Ag+ and Ag2O reduction takes place at a slower rate under base-free condition. In fact, Ag2O particles are more easily reduced than Ag+ due to a lower electrode potential (Ag+/Ag0: E0 = 0.7996 V; Ag2O/Ag0 + OH−: E0 = 0.342 V).30 AgNPs also are formed through the formation of ascorbate radicals (eqn (2) and (3)). It is in agreement with a minor reduction in solution pH after the synthesis (Fig. S4 of ESI†). The free radical character of organic compounds also eases Ag2O formation at a lower pH.1 Therefore, a bimodal distribution of AgNPs-pH 3 and AgNPs-pH 5 was observed (Fig. 3).
| 2Ag+ + 2OH− ↔ 2AgOH ↔ Ag2O + H2O | (1) |
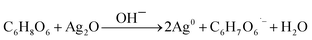 | (2) |
| 2C6H7O6˙− + Ag2O + H+ → ascorbate anion + dehydro-AA + 2Ag0 + H2O | (3) |
The variation of the zeta-potential (ζ) of AgNPs was determined to study its destabilization tendency in the aqueous media. ζ was negative for all the particles irrespective to the pH of synthesis (Fig. S5 of ESI†). The negative surface charge mostly resulted from the adsorption of biomolecules such as the formation of AgNPs–ascorbate layer.31 ζ gradually decreased with the increase in pH from 3 to 12.5. In the case of AgNPs-pH 3, ζ was found to decrease from −3.8 to −25.8 mV between pH 3 and 11 and, it varied from −22.1 to −86.2 mV for AgNPs-pH 12.5. It implies that AgNPs-pH 12.5 promoted destabilization compared to the particles formed at a lower pH. Moreover, the concentration of silver–ascorbate layer increases with the increase in pH. Hence, there may be better passivation of the nanocrystals surface32
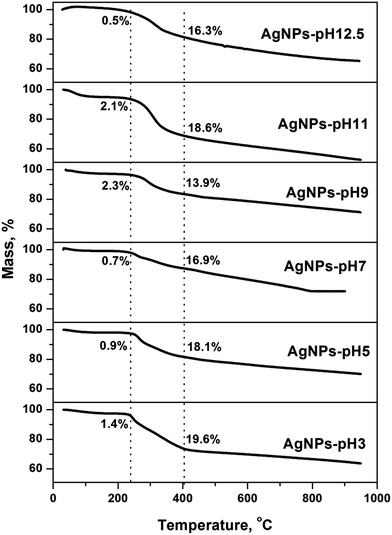
The presence of biomolecules on the surface of AgNPs was confirmed by TGA (Fig. 5). The mass losses took place in three distinct temperature regions (Fig. S6 of the ESI†). The mass loss at about 100 °C was due to the evaporation of adsorbed water molecules. There was about 20% mass loss at 200–390 °C resulted from the degradation of organic constituents, like AA, ferulic acid, carbohydrates, quercetins, and lignin molecules.21 The third steady mass loss was about 15% at 370–950 °C. It may be for the removal of bio-organic salt like carbonates and decomposition of resistive aromatic complexes.33 An average overall mass loss of 32.6 ± 4% was found within the whole temperature range. Shahzad et al.34 observed 16% mass loss up to 410 °C for AgNPs synthesized using branched polyethyleneimine. Sun et al.21 reported 19% mass loss at 380 °C and, it increased to 39% at 1000 °C for AgNPs synthesized using the tea extract.
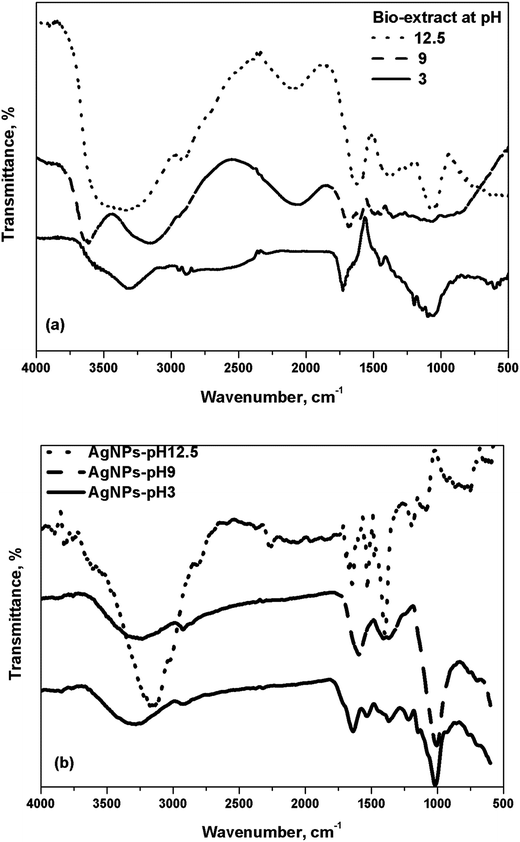
FTIR spectra supported the variation of the functional groups of the biomolecules involved in AgNPs formation and, the effect of pH variation in the absence of metal precursor is shown in Fig. 6(a). The bio-extract at different pH showed important peaks at 3312–3337, 2885–2889, 2285–2293, 1668–1683, 1444–1446, 1361–1373, 1090–1130, and 780–870 cm−1 wavenumber. The distinct broad peaks at around 3300 and 1675 cm−1 correspond to the phenolic–OH groups.35 The peak at around 3313 cm−1 at pH 3 was shifted to 3157 and 3298 cm−1 at pH 9 and 12.5, respectively. The positioning of this band relies upon the degree of the intensity of hydrogen bonding and, it moves to the lower wavenumbers when H–O–H bond is dominant.36 The bands at around 1614, 1365, and 1130 cm−1 attributed to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, C–O, and O–C–C bonds of AA32 and, the band intensity was more at a higher pH.
O, C–O, and O–C–C bonds of AA32 and, the band intensity was more at a higher pH.
AgNPs displayed a gradual shift of the peak position to a lower wavenumber with the rise in the intensities with pH elevation (Fig. 6(b)). It implies to hydrogen–AA bond breaking and formation of ascorbate ions.32 The peaks at 1722, 1602, and 1614 cm−1 at pH 3, 11, and 12.5 (Fig. 6(a)), respectively, correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of the five-membered lactone ring of AA. It shifted to 1641, 1600, and 1643 cm−1 in the case of AgNPs-pH 3, AgNPs-pH 11, and AgNPs-pH 12.5 (Fig. 6(b)) as the –C
O bond of the five-membered lactone ring of AA. It shifted to 1641, 1600, and 1643 cm−1 in the case of AgNPs-pH 3, AgNPs-pH 11, and AgNPs-pH 12.5 (Fig. 6(b)) as the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of the lactone ring primarily bound on AgNPs.37 The bands appeared at around 1377, 1400, and 1406 cm−1 for AgNPs-pH 3, AgNPs-pH9, and AgNPs-pH 12.5, respectively, with the dominance for AgNPs-pH 12.5 owing to the C
O group of the lactone ring primarily bound on AgNPs.37 The bands appeared at around 1377, 1400, and 1406 cm−1 for AgNPs-pH 3, AgNPs-pH9, and AgNPs-pH 12.5, respectively, with the dominance for AgNPs-pH 12.5 owing to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of ascorbate ion.32 So, the formation of silver–ascorbate layer (capping layer) was significant at a higher pH.
O bond of ascorbate ion.32 So, the formation of silver–ascorbate layer (capping layer) was significant at a higher pH.
The XRD patterns of the particles formed at various pH are shown in Fig. 7. It corresponds to the fcc AgNPs crystal. The major peaks at 38, 46, 65, and 78° attribute to (1 1 1), (2 0 0), (2 2 0), and (3 1 1) planes, respectively.3 The crystallite size was calculated by the Scherrer's formula at 2θ = 38° and, the reduction in crystallite size was more till pH 7 and, the raise was minor even at a higher pH (Table S1 of the ESI†). The crystallite size of 54.1 nm for AgNPs-pH 3 decreased to 21.7 nm for AgNPs-pH 12.5 due to peak broadening. However, the d-spacing between the adjacent lattice planes didn't change with the pH variation (Table S1 of ESI†). A minor peak at 2θ = 32.2° (Fig. 7) for AgNPs-pH 3 and AgNPs-pH 12.5 corroborates the existence of Ag2O. Thus, AgNPs were bi-crystalline both at a very low and high pH. Similar results are also found for AgNPs synthesized with the tea leaf extract.21
3.2. Effect of AgNPs on antimicrobial activity
The bacterial growth kinetics was studied using the spectrophotometric technique at different doses of AgNPs-pH 9. Two bacterial strains, i.e., B. subtilis and E. coli were tested. Fig. 8(a) and (b) show the bacterial growth inhibition in the presence of AgNPs-pH 9 and its comparison with the control media. The rate of inhibition increased gradually with the increase in particles concentration. Both B. subtilis and E. coli with AgNPs-pH 9 ≥ 25 μg mL−1 exhibited the complete growth inhibition. The growth didn't propagate with E. coli, so, the lag phase appeared early. In the case of B. subtilis, the stationary phase could not be visualized distinctly. However, the stationary phase was clearly distinguishable with E. coli due to a higher growth inhibition.25 2, 5, and 10 μg mL−1 AgNPs-pH 9 caused 6, 34, and 45% decrease in B. subtilis growth compared to the control media. E. coli at the same concentration of AgNPs-pH 9 showed 12, 45, and 68% reduction in the cell density. A Gram-negative bacterium has a protective peptidoglycan membrane whereas a Gram-positive bacterium doesn't have it. AgNPs owning to free radical character can break the membrane lipids1 resulting in the damage of cellular materials (Fig. 9). The g-factor varied from 2.0052 to 2.0058 which is close to ascorbate free radical (eqn (2)).38 Usually, the higher surface area of smaller particles interacts more with the bacteria leading to a higher antimicrobial activity.12,23 Similar results were also obtained in this work for AgNPs with different particles sizes (Fig. S7 of the ESI†).25,39 | ||
| Fig. 8 Bacterial growth in terms of optical density measurements against doses of AgNPs-pH 9 at a wavelength of 600 nm for (a) B. subtilis and (b) E. coli. | ||
It was also noticed that the illumination of visible light (60 W, Bajaj, India) improved the antimicrobial activity of AgNPs (Fig. 8). The growth inhibition was even faster in the presence of light. Tahir et al.40 found the complete growth inhibition of E. coli within 4 h using AgNPs (15–40 nm) under the visible light illumination, but it took 24 h in the absence of light.
3.3. AA stability and mechanism of AgNPs formation
The effect of pH on the stability of AA is shown in Fig. S8 of the ESI.† AA concentration was found to be 170 mg L−1 at pH 5.8 (natural extract pH). There was a gradual decay in AA concentration in the bio-extract with the decrease in pH, but, it increased at a higher pH (5.8 < pH < 9). AA is converted to dehydro-AA through a free radical intermediate in a reversible process at mild acidic and neutral pH.41,42 However, the inter-conversion is irreversible at a higher pH.43 These results are in line with a slightly higher concentration of residual AA in the extract after AgNPs synthesis at a higher pH (Fig. S8 of the ESI†). AA (m/z 175) didn't appear in the mass spectra of the bio-extract after AgNPs formation at pH 3 but dehydro-AA (m/z 174.1) was identified (Fig. S9 of the ESI†). AA, as a sacrificial electron donor, reduces Ag2O (or Ag+) and, dehydro-AA or 2,3-diketogulonic acid (2,3-DKG, m/z 192) is formed (eqn (4)).44 2,3-DKG appeared in the spectra at pH 9 and 12.5. The fragments with m/z of 127, 147.9, and 165.9 may be of oxalic acid, xylosone, and xylonic acid, respectively.44,45 Dehydro-AA, 2,3-DKG, oxalic, pyruvic, glyceric, and threonic acids were detected in the mass spectra which are usually the degradation products of AA.44 Zhang et al.45 found that the oxidation of AA to 2,3-DKG can yield the fragments like pyruvic acid (m/z 87.01), glyceric acid (m/z 104.1), and threonic acid (m/z 134) from the degradation of 2,3-DKG during synthesis of palladium nanoparticles. Flavonoids present in this bio-extract also could be responsible for the formation of AgNPs9,46 but, proteins usually provide the stability of the particles as it act as the capping agents.47
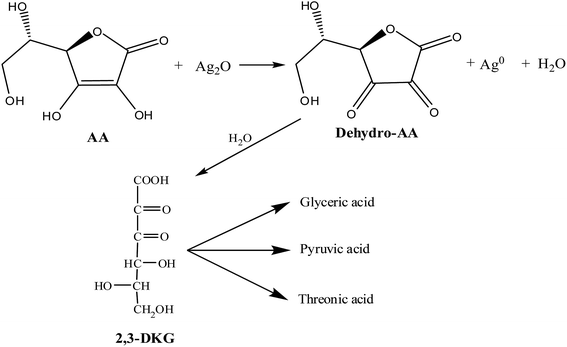 | (4) |
4. Conclusions
Bio-inspired synthesis of AgNPs using the aqueous bio-extract of Sechium edule is strongly pH dependent which in turn determines the size, shape, self-stabilization, and purity of particles and, its mechanism of formation. pH also controls the concentration of AA present in the bio-extract. However, the residual AA concentration didn't vary significantly after the formation of AgNPs at different pH. AgNPs were formed through the reduction of both Ag+ and Ag2O; but, the smaller particles of nearly spherical shape were found at a higher pH with a faster kinetics where Ag2O is abundant. A gradual blue shift in the SPR peak at around 425 nm was observed with the decrease in particle sizes with the increase in pH of AgNPs formation. The intense negative zeta-potential and formation silver–ascorbate layer on AgNPs at a higher pH facilitated the prevention of particles aggregation. The average hydrodynamic diameters were 68, 60, 45, 31, 15, and 9 nm at pH 3, 5, 7, 9, 11, and 12.5, respectively. AgNPs exhibited the character of ascorbate free radicals which might cause the synergistic effects in B. subtilis and E. coli inactivation mechanisms.Acknowledgements
We gratefully thank Indian Institute of Technology Guwahati, for providing the research fellowship to Mr Venkatanarasimha Rao Chelli and, the necessary research facilities to the Department of Chemical Engineering, and Central Instruments Facility (CIF). We are also grateful to Department of Science and Technology for providing the grant under FIST scheme (SR/FST/ET11-047/2010) to the Department of Chemical Engineering at Indian Institute of Technology Guwahati.References
- N. F. Adegboyega, V. K. Sharma, K. Siskova, R. Zbořil, M. Sohn, B. J. Schultz and S. Banerjee, Environ. Sci. Technol., 2013, 47, 757–764 CrossRef CAS PubMed.
- A. M. E. Badawy, T. P. Luxton, R. G. Silva, K. G. Scheckel, M. T. Suidan and T. M. Tolaymat, Environ. Sci. Technol., 2010, 44, 1260–1266 CrossRef PubMed.
- O. Zuas, N. Hamim and Y. Sampora, Mater. Lett., 2014, 123, 156–159 CrossRef CAS.
- S. Ahmed, M. Ahmad, B. L. Swami and S. Ikram, J. Adv. Res., 2016, 7, 17–28 CrossRef CAS PubMed.
- Z. G. Wu, M. Munoz and O. Montero, Adv. Powder Technol., 2010, 21, 165–168 CrossRef CAS.
- M. Ghorbani, H. Abdizadeh and M. R. Golobostanfard, Procedia Mater. Sci., 2015, 11, 326–330 CrossRef CAS.
- Y. Tan, Y. Li and D. Zhu, J. Colloid Interface Sci., 2003, 258, 244–251 CrossRef CAS PubMed.
- Z. Khan, S. A. Al-Thabaiti, A. Y. Obaid and A. O. Al-Youbi, Colloids Surf., B, 2011, 82, 513–517 CrossRef CAS PubMed.
- C. V. Rao and A. K. Golder, Colloids Surf., A, 2016, 506, 557–565 CrossRef CAS.
- A. L. B. Shimada, A. Lino-dos-Santos-Franco, S. M. Bolonheis, A. Nakasato, A. S. Damazo, W. Tavares-de-Lima and S. H. P. Farsky, Toxicol. Lett., 2012, 211, 10–17 CrossRef CAS PubMed.
- L. Yang, Y. Shen, A. Xie and B. Zhang, J. Phys. Chem. C, 2007, 111, 5300–5308 CAS.
- M. D. Balakumaran, R. Ramachandran and P. T. Kalaichelvan, Microbiol. Res., 2015, 178, 9–17 CrossRef CAS PubMed.
- S. Ahmed, Saifullah, M. Ahmad, B. L. Swami and S. Ikram, J. Radiat. Res. Appl. Sci., 2016, 9, 1–7 CrossRef.
- S. M. Roopan, Rohit, G. Madhumitha, A. A. Rahuman, C. Kamaraj, A. Bharathi and T. V. Surendra, Ind. Crops Prod., 2013, 43, 631–635 CrossRef CAS.
- S. Pandey, A. Mewada, M. Thakur, S. Shinde, R. Shah, G. Oza and M. Sharon, J. Nanosci., 2013, 2013, 1–9 CrossRef.
- M. Friedman and H. S. Jurgens, J. Agric. Food Chem., 2000, 48, 2101–2110 CrossRef CAS PubMed.
- K. K. Chebrolu, G. K. Jayaprakasha, K. S. Yoo, J. L. Jifon and B. S. Patil, LWT--Food Sci. Technol., 2012, 47, 443–449 CrossRef CAS.
- G. Singhal, R. Bhavesh, K. Kasariya, A. R. Sharma and R. P. Singh, J. Nanopart. Res., 2011, 13, 2981–2988 CrossRef CAS.
- T. C. Prathna, N. Chandrasekaran, A. M. Raichur and A. Mukherjee, Colloids Surf., B, 2011, 82, 152–159 CrossRef CAS PubMed.
- R. Konwarh, B. Gogoi, R. Philip, M. A. Laskar and N. Karak, Colloids Surf., B, 2011, 84, 338–345 CrossRef CAS PubMed.
- Q. Sun, X. Cai, J. Li, M. Zheng, Z. Chen and C. P. Yu, Colloids Surf., A, 2014, 444, 226–231 CrossRef CAS.
- P. Sutradhar and M. Saha, J. Phys. Chem. C, 2016, 120, 8941–8949 CAS.
- B. Ajitha, Y. A. K. Reddy and P. S. Reddy, Powder Technol., 2015, 269, 110–117 CrossRef CAS.
- M. Sathishkumar, K. Sneha, S. W. Won, C.-W. Cho, S. Kim and Y.-S. Yun, Colloids Surf., B, 2009, 73, 332–338 CrossRef CAS PubMed.
- S. Agnihotri, S. Mukherji and S. Mukherji, RSC Adv., 2014, 4, 3974–3983 RSC.
- S. L. Smitha, K. M. Nissamudeen, D. Philip and K. G. Gopchandran, Spectrochim. Acta, Part A, 2008, 71, 186–190 CrossRef CAS PubMed.
- J. I. E. T. N. Edison and M. G. Sethuraman, ACS Sustainable Chem. Eng., 2013, 1, 1326–1332 CrossRef.
- M. Yilmaz, H. Turkdemir, M. A. Kilic, E. Bayram, A. Cicek, A. Mete and B. Ulug, Mater. Chem. Phys., 2011, 130, 1195–1202 CrossRef CAS.
- V. R. Chelli, S. S. Bag and A. K. Golder, Environ. Prog. Sustainable Energy, 2016, 1–8, DOI:10.1002/ep.
- Y. Cai, F. Tan, X. Qiao, W. Wang, J. Chen and X. Qiu, RSC Adv., 2016, 6, 18407–18412 RSC.
- Y. Zhang, Y. Chen, P. Westerhoff and J. Crittenden, Water Res., 2009, 43, 4249–4257 CrossRef CAS PubMed.
- O. S. Oluwafemi, N. Revaprasadu and O. O. Adeyemi, Colloids Surf., B, 2010, 79, 126–130 CrossRef CAS PubMed.
- T. Carballo, M. V. Gil, X. Gomez, F. Gonzalez-Andres and A. Moran, Biodegradation, 2008, 19, 815–830 CrossRef PubMed.
- A. Shahzad, W.-S. Kim and T. Yu, RSC Adv., 2015, 5, 28652–28661 RSC.
- B. Sadeghi, M. Mohammadzadeh and B. Babakhani, J. Photochem. Photobiol., B, 2015, 148, 101–106 CrossRef CAS PubMed.
- U. Thanganathan, in Materials Challenges in Alternative and Renewable Energy II: Ceramic Transactions, John Wiley & Sons, Inc., 2013, pp. 215–221 Search PubMed.
- V. Sreeja, K. N. Jayaprabha and P. A. Joy, Appl. Nanosci., 2015, 5, 435–441 CrossRef CAS.
- R. G. Buettner and B. A. Jurkiewicz, Free Radical Biol. Med., 1993, 14, 49–55 CrossRef PubMed.
- J. P. Ruparelia, A. K. Chatterjee, S. P. Duttagupta and S. Mukherji, Acta Biomater., 2008, 4, 707–716 CrossRef CAS PubMed.
- K. Tahir, S. Nazir, B. Li, A. U. Khan, Z. U. H. Khan, A. Ahmad, Q. U. Khan and Y. Zhao, J. Photochem. Photobiol., B., 2015, 153, 261–266 CrossRef CAS PubMed.
- C. Bachmann, B. Probst, M. Guttentag and R. Alberto, Chem. Commun., 2014, 50, 6737–6739 RSC.
- J. Fenoll, A. Martínez, P. Hellín and P. Flores, Food Chem., 2011, 127, 340–344 CrossRef CAS.
- L. Wechtersbach and B. Cigic, J. Biochem. Biophys. Methods, 2007, 70, 767–772 CrossRef CAS PubMed.
- M. Smuda and M. A. Glomb, Angew. Chem., Int. Ed., 2013, 52, 4887–4891 CrossRef CAS PubMed.
- A. Zhang, M. Liu, M. Liu, Y. Xiao, Z. Li, J. Chen, Y. Sun, J. Zhao, S. Fang, D. Jia and F. Li, J. Mater. Chem. A, 2014, 2, 1369–1374 CAS.
- N. Sahu, D. Soni, B. Chandrashekhar, D. B. Satpute, S. Saravanadevi, B. K. Sarangi and R. A. Pandey, Int. Nano Lett., 2016, 6, 173–181 CrossRef.
- S. Mandal, S. Phadtare and M. Sastry, Curr. Appl. Phys., 2005, 5, 118–127 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: ESI file includes a table consisting of the characteristic parameters of AgNPs, and nine graphical illustrations. The figures include UV-vis spectra of bio-extract, FESEM micrographs, XRD spectra of Ag2O, pH variation, zeta potential of AgNPs, TGA-DTG, antimicrobial activity by disc diffusion method, AA content in bio-extract, and mass spectra of bio-extract at different pH. See DOI: 10.1039/c6ra16475g |
| This journal is © The Royal Society of Chemistry 2016 |