Aggregation behavior of N-alkyl imidazolium-based poly(ionic liquid)s in an organic solvent†
Qing Ge,
Dingshuo Lou,
Shui Lu,
Wei Zhang*,
Li Zhang and
Xinping Wang
Department of Chemistry, Key Laboratory of Advanced Textile Materials and Manufacturing Technology of Education Ministry, Zhejiang Sci-Tech University, Hangzhou 310018, China. E-mail: zhwei@zstu.edu.cn; zwzist@163.com
First published on 25th August 2016
Abstract
Poly(ionic liquid)s (PILs) have promising potential to form assemblies with ordered structures in organic media, but experimental investigation on the detailed aggregation behavior of PILs has seldom been involved. In this study, a series of N-alkyl imidazolium-based PILs with different chemical composition in their n-propanol solution was investigated by conductivity measurements. TEM images indicate that PILs with long alkyl chains exhibit onion-like multilamellar structures. The thermodynamic parameters suggest that aggregation of PILs in their good solvents (n-PrOH) is spontaneous, driven by entropy change associated with a hydrophobic effect. As increasing alkyl chain length from 8 to 16, the contribution of per alkyl chain to ΔGθm for PIL aggregation is found to decrease by more than 2 times. The nonsolvent (H2O) contribution to change of Gibbs free energy  as well as critical aggregation concentration (CAC) decrease linearly with the volume fraction of water in mixed solvents, implying that PIL aggregation becomes more favorable with the addition of nonsolvent. The results mentioned above play an important role in understanding the aggregation process of PIL in an organic solvent and developing functional materials with well-ordered structures.
as well as critical aggregation concentration (CAC) decrease linearly with the volume fraction of water in mixed solvents, implying that PIL aggregation becomes more favorable with the addition of nonsolvent. The results mentioned above play an important role in understanding the aggregation process of PIL in an organic solvent and developing functional materials with well-ordered structures.
1. Introduction
Ionic liquids (ILs) are a class of molten organic salts with melting points below 100 °C or even lower than room temperature.1 Recently, they have attracted considerable attention because of their chemical stability, low flammability, negligible vapor pressure, high ionic conductivity, and wide electrochemical window. Moreover, ILs are generally composed of a variety of cations and anions, which endow them with diverse structures and properties.2N-Alkyl methylimidazolium-based ILs can form aggregates in their aqueous solution and act as a cationic surfactant.3 In particular, the surface activity of N-alkyl methylimidazolium-based ILs is found to be superior to that of conventional ionic surfactants as longer alkyl chains are attached to the imidazolium ring.4 For example, the imidazolium ILs, CnmimBr (n = 14, 16), display lower critical micelle concentration (CMC) and smaller micelle aggregation numbers when compared to the corresponding cationic surfactants, alkyl trimethylammonium bromides (CnTAB).5 The excellent surface activity of N-alkyl imidazolium-based ILs drives progressive investigation of their aggregation behavior in an aqueous solution. The increase in the alkyl tail length is generally found to reduce the standard Gibbs free energy of aggregation, and hence favors the self-aggregation of N-alkyl imidazolium-based ILs.6,7 The anion also has a significant effect on the aggregation process of ILs. It was reported that the micellization of [C12min]Cl and [C12min]Br was entropy-driven, whereas that of [C12min]I was enthalpy-driven at 15 °C but entropy-driven above 20 °C.8 In addition, the dependence of aggregation behavior of ILs on salt additives and temperature is well established.9–11
Poly(ionic liquid)s (PILs), or polymerized ILs, have emerged as a new class of materials that combine the unique properties and structural designability of ILs with mechanical stability and improved processability of polymers, and they have exhibited promising application in the field of functional materials such as solid state polyelectrolytes,12 CO2 sorbents,13,14 dispersants,15 porous materials,16,17 microwave-absorbing materials,18 and catalysts.19 Interestingly, the vinyl imidazolium-type IL monomers with long alkyl chains could perform an aqueous dispersion polymerization without added stabilizers because these IL monomers and their oligomers are effective dispersants.20 The as-prepared PIL nanoparticles display multilamellar or unilamellar vesicular morphology and have been demonstrated to stem from a simultaneous polymerization/self-assembly process.21 In addition, adsorption experiments of poly[1-(11-acryloyloxyundecyl)-3-methylimidazolium bromide] (PILBr) at the air–water interface indicate that PILBr is surface active at a quite low concentration and the interfacial area density is about 56 Å2 per monomer.22
Obviously, PILs with longer alkyl tails are surface active and can act as surfactants, which is similar to the nature of ILs. Although the aggregation process of ILs in an aqueous solution is well established, the investigation on aggregation behavior of PILs has seldom been involved. On the other hand, PILs with different types of anions and cations often dissolve in an organic solvent, and even many PIL assemblies with ordered structures are formed from their organic media, thus the physicochemical properties of PIL solution in an organic solvent are of vital importance for understanding the self-aggregation and ordered packing of PILs. Therefore, in this study, we focus on the aggregation behavior of PILs in an organic solvent, particularly on the effect of the length of alkyl chains and solvent quality. For this purpose, a series of N-alkyl imidazolium-based PILs with different alkyl chain length, as shown in Scheme 1, were prepared by a common solution polymerization. After normal dissolution–precipitation purification, the PILs were dissolved in their organic solvent, and the sizes and morphologies of the formed aggregates were explored in terms of alkyl chain length. The thermodynamic parameters (ΔGθm, ΔHθm, ΔSθm) for the aggregates formation were derived from the temperature dependence of critical aggregation concentration (CAC) and the concentration dependence of conductivity. The effect of alkyl chain length and solvent quality on thermodynamic parameters was discussed in detail. The results might play an important role in the self-assembly of PILs and their promising application such as in the fabrication of ordered structures.
2. Experimental
2.1 Materials
1-Vinylimidazole (Aldrich, 99%), 1-bromooctane (Aldrich, 99%), 1-bromododecane (Aldrich, 99%), 1-bromohexadecane (Aldrich, 99%), azoisobutyronitrile (AIBN, Aldrich, 98%), bis(trifluoromethane) sulfonamide lithium salt (LiTf2N, Aladdin, 99%), and NaBF4 (Aladdin, 99%) were used as received. All reagents such as acetone, n-propanol (n-PrOH), chloroform, ethyl acetate and tetrahydrofuran (THF) were of analytical grade and were used without further purification.2.2 Synthesis of ionic liquid monomers
Ionic liquid monomers were synthesized as described in the literature.17 In a 100 mL flask, 0.1 mol of 1-vinylimidazole, 0.1 mol of n-alkyl bromide and 30 mL of dry ethanol were introduced. The mixture was stirred vigorously for 24 h at 70 °C under nitrogen atmosphere. The obtained viscous liquid was washed with excess of ethyl acetate, dried under vacuum at 40 °C, and then stored in a refrigerator. The product, 1-octyl-3-vinylimidazolium bromide ([C8VIm+][Br−]), was a pale yellow liquid with a yield of 80%, whereas the products of 1-dodecyl-3-vinylimidazolium bromide ([C12VIm+][Br−]) and 1-hexadecyl-3-vinylimidazolium bromide([C16VIm+][Br−]) were white powders with yields of 78% and 76%, respectively.2.3 Polymerization process
The samples of PILs were synthesized via free radical polymerization. In a dry 250 mL round-bottomed flask, 5 g of [CnVIm+][Br−] (n = 8, 12, 16), certain amount of AIBN (molar ratio of AIBN and [CnVIm+][Br−] was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100) and 30 mL of ethanol were mixed. The mixtures were sealed and completely degassed. The flask was then stirred in an oil bath at 65 °C for 24 h under nitrogen atmosphere. After polymerization, the mixture was precipitated into acetone, and then washed with fresh acetone several times. The products were dried under vacuum at 40 °C. The physical state, yield and molecular weight of poly[CnVIm+][Br−] are listed in Table S1.† Poly[C8VIm+][Br−] was a yellow powder, whereas poly[C12VIm+][Br−] and poly[C16VIm+][Br−] were white powders.
100) and 30 mL of ethanol were mixed. The mixtures were sealed and completely degassed. The flask was then stirred in an oil bath at 65 °C for 24 h under nitrogen atmosphere. After polymerization, the mixture was precipitated into acetone, and then washed with fresh acetone several times. The products were dried under vacuum at 40 °C. The physical state, yield and molecular weight of poly[CnVIm+][Br−] are listed in Table S1.† Poly[C8VIm+][Br−] was a yellow powder, whereas poly[C12VIm+][Br−] and poly[C16VIm+][Br−] were white powders.
2.4 Characterization methods
It should be pointed out that an unreliable MW characterization is normally obtained via GPC due to the presence of charged groups in PILs. Simple addition of LiTf2N into the eluent such as THF can overcome this difficulty.23 Therefore, we changed the anions of PILs from Br− to LiTf2−, and then conducted the GPC measurement using THF containing 10 mM LiTf2N as the eluent at a flow rate of 0.8 mL min−1. The column temperature was 30 °C. A series of polystyrene (PS) standards with molecular weights ranging from 1190 to 295![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 were employed to generate the calibration curve. The MW of poly[CnVIm+][Br−], as listed in Table S1,† is calculated from GPC results of the corresponding poly[CnVIm+][Tf2N−].
000 were employed to generate the calibration curve. The MW of poly[CnVIm+][Br−], as listed in Table S1,† is calculated from GPC results of the corresponding poly[CnVIm+][Tf2N−].
3. Results and discussion
3.1 Morphology of PILs aggregates from the solution in organic solvents
It was reported that PIL nanoparticles with lamellar structures formed in the aqueous dispersion polymerization of IL monomer, and further characterization revealed that the PILs spontaneously self-assembled into this ordered structure during polymerization.21 In an effort to clarify what type of structures are presented in the PILs/organic solvent system, we synthesized a series of PILs (poly[CnVIm+][Br−] (n = 8, 12, 16)) and explored their aggregate morphologies in an organic solvent. Note that n-PrOH was chosen as an organic solvent because all the PILs were well dissolved in n-PrOH (the solubility of PILs in different solvents is shown in Table S2†).The PIL aggregates cast from their n-PrOH solution were characterized using room-temperature transmission electron microscopy (TEM). Poly[CnVIm+][Br−] (n = 8, 12) aggregates seem to be sphere-like particles (Fig. 1a and b), whereas poly[C16VIm+][Br−] displays an onion-like multilamellar structure, as shown in Fig. 1c and d. In order to verify the effect of counterion on aggregate morphology, we varied the anion from Br− to BF4− by a typical anion exchange process.24 A similar onion-like morphology is observed for poly[C16VIm+][BF4−] aggregates, as shown in Fig. 1e and f, implying that the morphologies of PIL aggregates are independent of their counteranions. In particular, there is almost no change in the multilamellar morphology for the samples of poly[C16VIm+][X−] (X = Br−, BF4−) when their casting solution has been stored for 7 days (Fig. 1d, g and f, h), indicative of a desirable stability. It should be pointed out that in these onion-like structures, the dark rings normally correspond to the charge pairs of imidazolium cation and the X anion due to the high contrast of the X atoms under the electron beam, whereas the close-to-transparent space between neighbouring rings results from the alkyl chains that are ordered as lamellae.25 The incompatibility between the hydrophobic domains formed by the alkyl tails and the hydrophilic domains formed by the imidazolium cation and the Br− anion becomes more pronounced when the alkyl chain length is increased in order to reduce the thermodynamically unfavorable stretching of the longer alkyl chains. Therefore, only the poly[C16VIm+][X−] (X = Br−, BF4−) samples exhibit highly ordered multilamellar structures, which in fact results from the self-assembly behavior of PILs themselves.21
Furthermore, DLS results (Fig. 2) indicate that the hydrodynamic radius of the aggregates increases slightly when the length of the alkyl chain varies from 8 to 12, but enhances significantly with further increasing alkyl chain length. For example, the average sizes of poly[C16VIm+][Br−] aggregates are 0.7–0.8 μm in diameter, much larger than those of poly[CnVIm+][Br−] (n = 8, 12). The larger PIL aggregates are due to the fact that long alkyl chains tend to fully expand in an effort to decrease the interfacial area between the aggregates and the solvent.26
 | ||
| Fig. 2 (a) Size distributions of poly[CnVIm+][Br−] in n-PrOH solutions; (b) plot of diameter versus length of alkyl chains of poly[CnVIm+][Br−]. | ||
3.2 Thermodynamics of PIL aggregation in an organic solvent
 | ||
| Fig. 3 Plots of electrical conductivity, κ, against concentration of (a) poly[C8VIm+][Br−], (b) poly[C12VIm+][Br−], (c) poly[C16VIm+][Br−] at different temperatures. | ||
Compared to CAC, on the other hand, β values of PILs have different dependence on the length of alkyl chains. As shown in Fig. 4b, β values show almost no noticeable change with the increase of alkyl chain length. This suggests that the ability of the counterion to bind to aggregates appears to be independent of the length of alkyl chain. In fact, the polarizability and cavitational force of counteranions contribute much more to β values,29 and the effect of alkyl chains is less pronounced.
 | (1) |
ΔHθm can be obtained from the Gibbs–Helmholtz equation:
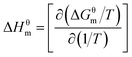 | (2) |
Eqn (3) is deduced by substituting eqn (1) into eqn (2):
ΔHθm = −(1 + β)RT2dln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) XCAC/dT XCAC/dT
| (3) |
Finally, the standard entropy change during the formation of aggregations is calculated by the following equation:
 | (4) |
In order to estimate the effect of alkyl chains on the aggregation process, the change in Gibbs free energy per alkyl chain is calculated through the following equation31 in which n is the polymerization degree of PILs (see Table S1†).
 | (5) |
Table 1 shows the calculated thermodynamic parameters for poly[C8VIm+][Br−], poly[C12VIm+][Br−], poly[C16VIm+][Br−] at different temperatures. It is clear that ΔGθm values for all poly[CnVIm+][Br−] samples are negative, suggesting that the aggregation of PILs is a spontaneous process. Moreover, the ΔGθm values for PILs vary between −40 and −50 kJ mol−1, which is more negative than those (generally higher than −40 kJ mol−1) for imidazolium-based ILs,6–9,32 ionic surfactants11 and non-ionic surfactants,33 implying that PIL aggregation is a more spontaneous process than IL aggregation. It should be pointed out that the ΔGθm for PIL aggregation exhibits a typical alkyl chain dependence. The change in Gibbs free energy per alkyl chain, ΔGθm,alkyl values become more negative with the increase in alkyl chain length. At 298.15 K, as the length of alkyl chains varies from 8 to 16, the contribution per alkyl chain to the change in Gibbs free energy decreases from −0.69 to −1.65 kJ mol−1, indicative of a more than 2-fold decrease. The results mentioned above suggest that the aggregation of PILs is mainly caused by the hydrophobic effect of alkyl chains.
| T (K) | ΔGθm (kJ mol−1) | ΔGθm,alkyl (kJ mol−1) | ΔHθm (kJ mol−1) | TΔSθm (kJ mol−1) | |
|---|---|---|---|---|---|
| poly[C8VIm+][Br−] | 298.15 | −40.78 | −0.69 | 6.41 | 47.19 |
| 303.15 | −42.08 | −0.72 | 6.59 | 48.67 | |
| 308.15 | −44.55 | −0.76 | 6.70 | 51.25 | |
| 313.15 | −45.93 | −0.78 | 6.88 | 52.81 | |
| 318.15 | −47.39 | −0.81 | 6.92 | 54.31 | |
| poly[C12VIm+][Br−] | 298.15 | −43.47 | −1.21 | 10.61 | 54.08 |
| 303.15 | −44.49 | −1.23 | 10.85 | 55.34 | |
| 308.15 | −45.92 | −1.27 | 11.19 | 57.11 | |
| 313.15 | −47.15 | −1.31 | 11.49 | 58.64 | |
| 318.15 | −48.76 | −1.35 | 11.62 | 60.38 | |
| poly[C16VIm+][Br−] | 298.15 | −44.76 | −1.65 | 15.93 | 60.69 |
| 303.15 | −45.84 | −1.69 | 16.27 | 62.11 | |
| 308.15 | −47.20 | −1.74 | 16.61 | 63.81 | |
| 313.15 | −49.19 | −1.81 | 16.99 | 66.18 | |
| 318.15 | −51.37 | −1.89 | 17.04 | 68.41 |
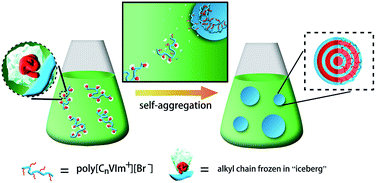
In a self-aggregation process, amphiphilic molecules can spontaneously change their structural states from monomeric molecules into aggregates, even assemblies with ordered structures, where the entropy change is inherently negative. As shown in Table 1, however, the ΔSθm values for poly[CnVIm+][Br−] (n = 8, 12, 16) aggregation are positive over the range of experimental temperatures. This is reasonable when the entropy change of the alkyl chains and solvent molecules during aggregation is taken into account. In a PIL solution, as shown in Scheme 2, the alkyl chains attached to PILs are frozen in “iceberg clusters”34 composed of n-PrOH molecules (a non-selective solvent for alkyl chains). Once self-aggregation occurs, the iceberg structures are destroyed, and the released alkyl chains form a hydrophobic layer. Obviously, the randomness of the alkyl chains locked in the “iceberg clusters” is less than that in the hydrophobic layer. On the other hand, the destruction of iceberg structures is also accompanied with an enhanced randomness for solvent molecules, indicative of an entropy-increased process. The contribution of the alkyl chains and solvent molecules results in a positive ΔSθm for PIL aggregates formation. It is interesting to note that as observed in aggregation of copolymers,35,36 ΔHθm values for all PIL samples are positive, implying that the aggregation is driven by entropy change associated with the hydrophobic effect of alkyl chains. This also indicates that the aggregation process of PILs in an organic solvent is spontaneous but endothermic.
3.3 Effect of solvent quality on PIL aggregation
Solvent quality is known to have significant influence on the aggregation process. Taking into account the desirable self-assembly behaviour of PILs with long alkyl chains, we investigated the aggregation behaviour of poly[C16VIm+][Br−] in n-PrOH containing nonsolvent water. Fig. 5a gives the DLS results of poly[C16VIm+][Br−] aggregates from the n-PrOH/H2O system. A clear relationship between aggregate size and water content in mixed solvents is shown in Fig. 5b. Note that the size of PIL aggregate from n-PrOH solution (the content of water in mixed solvent is zero) is obtained from Fig. 2. The average sizes in diameter for poly[C16VIm+][Br−] aggregates decrease remarkably when the volume fraction of water in mixed solvents increases from 0% to 10%, above which the decreasing trend seems less pronounced. The change in PIL aggregate sizes may be due to the collapse of PIL chains, resulting from the addition of a nonsolvent. It is notable that the mixtures of poly[C16VIm+][Br−] aggregates in the n-PrOH/H2O system exhibit desirable stability since an apparent Tyndall effect (Fig. 5b, inset) can be observed even though they have been stored for two months (no Tyndall effect was observed in poly[C16VIm+][Br−] n-PrOH solution). In addition, the aggregate morphology varies with the addition of water. In n-PrOH, poly[C16VIm+][Br−] aggregates display well-defined onion-like multilamellar structures (Fig. 1e and f), whereas PIL micelles are formed in the mixed solvents containing 20 vol% water (Fig. 5).The thermodynamic parameters and CACs for poly[C16VIm+][Br−] in n-PrOH/H2O mixed solvents are obtained from the conductivity data (Table S4 and Fig. S1†). As shown in Fig. 6 (also Table 2), the CACs decrease linearly with the content of nonsolvent, H2O, in the mixed solvents, indicating that the nonsolvent favors of PIL aggregation. In order to further estimate the nonsolvent dependence of aggregation, the contribution of nonsolvent (H2O) to the change of Gibbs free energy, 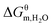 , is calculated using eqn (6):
, is calculated using eqn (6):
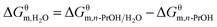 | (6) |
 | ||
Fig. 6 Free energy contribution from water addition ( ), and CAC ( ), and CAC ( ) for poly[C16VIm+][Br−] plotted vs. volume fraction of water in mixed solvents. ) for poly[C16VIm+][Br−] plotted vs. volume fraction of water in mixed solvents. | ||
| Water content in mixed solvents (vol%) | CAC (mol L−1) × 105 | β | (kJ mol−1) | ΔGθm,alkyl (kJ mol−1) | (kJ mol−1) |
|---|---|---|---|---|---|
| 0 | 4.61 | 0.44 | −44.76 | −1.65 | 0 |
| 7 | 4.31 | 0.54 | −49.64 | −1.84 | −4.88 |
| 8 | 3.98 | 0.55 | −50.24 | −1.86 | −5.48 |
| 9 | 3.68 | 0.55 | −50.79 | −1.88 | −6.03 |
| 10 | 3.37 | 0.54 | −51.09 | −1.89 | −6.33 |
| 15 | 1.32 | 0.53 | −55.12 | −2.04 | −10.36 |
| 20 | 0.67 | 0.49 | −56.25 | −2.08 | −11.49 |
As shown in Table 2, the  values vary between −49.64 and −56.25 kJ mol−1 over the experimental range of water contents, more negative than that (−44.76 kJ mol−1) for poly[C16VIm+][Br−] aggregation in n-PrOH. In addition,
values vary between −49.64 and −56.25 kJ mol−1 over the experimental range of water contents, more negative than that (−44.76 kJ mol−1) for poly[C16VIm+][Br−] aggregation in n-PrOH. In addition,  becomes more negative with the addition of water in mixed solvents, and a good linear relationship between
becomes more negative with the addition of water in mixed solvents, and a good linear relationship between  and volume fraction of water in mixed solvents is observed (Fig. 6), similar to the CAC trend. In order to further show the effect of nonsolvent, we added another nonsolvent, acetone, into n-PrOH and measured the electrical conductivity of poly[C16VIm+][Br−] in n-PrOH/acetone systems (Table S5 and Fig. S2†). According to these conductivity data, the thermodynamic parameters for poly[C16VIm+][Br−] in n-PrOH/acetone mixed solvents are obtained and listed in Table S6.† It is interesting to note that the addition of acetone has a similar effect on aggregation behaviour of PILs as the introduction of water. The cosolvent-induced free energy of transfer (ΔGθcosol) is also found to be more negative when more cosolvents (such as glycerol, glucose, and triacetin) are added into the aqueous solution of PEO–PPO–PEO block copolymers.37 The decrease in the change of Gibbs free energy suggests that PIL aggregation in a mixed solvent is more spontaneous than that in the individual good solvent. The self-assembly process of poly[C16VIm+][Br−] is normally driven by the different solubility between the hydrophobic domain and the hydrophilic one (as mentioned in Section 3.1) in n-PrOH. Obviously, the solubility of alkyl chains is dramatically decreased upon the addition of water since water is a typical nonsolvent for alkyl tails. In this case, the worsening of the solvent quality leads to a decrease in CAC and Gibbs free energy.38 In addition, the introduction of water reduces the contact between n-PrOH and the alkyl chains because of the strong interaction between water and n-PrOH, which expands the solubility difference between the formed hydrophobic domain and the hydrophilic one, and hence facilities PIL aggregation.
and volume fraction of water in mixed solvents is observed (Fig. 6), similar to the CAC trend. In order to further show the effect of nonsolvent, we added another nonsolvent, acetone, into n-PrOH and measured the electrical conductivity of poly[C16VIm+][Br−] in n-PrOH/acetone systems (Table S5 and Fig. S2†). According to these conductivity data, the thermodynamic parameters for poly[C16VIm+][Br−] in n-PrOH/acetone mixed solvents are obtained and listed in Table S6.† It is interesting to note that the addition of acetone has a similar effect on aggregation behaviour of PILs as the introduction of water. The cosolvent-induced free energy of transfer (ΔGθcosol) is also found to be more negative when more cosolvents (such as glycerol, glucose, and triacetin) are added into the aqueous solution of PEO–PPO–PEO block copolymers.37 The decrease in the change of Gibbs free energy suggests that PIL aggregation in a mixed solvent is more spontaneous than that in the individual good solvent. The self-assembly process of poly[C16VIm+][Br−] is normally driven by the different solubility between the hydrophobic domain and the hydrophilic one (as mentioned in Section 3.1) in n-PrOH. Obviously, the solubility of alkyl chains is dramatically decreased upon the addition of water since water is a typical nonsolvent for alkyl tails. In this case, the worsening of the solvent quality leads to a decrease in CAC and Gibbs free energy.38 In addition, the introduction of water reduces the contact between n-PrOH and the alkyl chains because of the strong interaction between water and n-PrOH, which expands the solubility difference between the formed hydrophobic domain and the hydrophilic one, and hence facilities PIL aggregation.
It can also be seen from Table 2 that the change in Gibbs free energy per alkyl chain, ΔGθm,alkyl, is more negative as water content increases in mixed solvents. Considering the results in Tables 1 and 2, one can conclude that the contribution of alkyl chains to PIL aggregation is related not only to the alkyl chain length, but also the solvent quality.
4. Conclusion
N-Alkyl imidazolium-based PILs with different lengths of alkyl chain were synthesized by a free radical solution polymerization. After normal dissolution–precipitation purification, the PILs were dissolved in their organic solvents. The obtained poly[CnVIm+][X−] (X = Br−, BF4−) aggregates exhibit onion-like multilamellar structures when longer alkyl chains (n = 16) are attached to the imidazolium ring. Formation of such ordered structure is due to the incompatibility between the hydrophobic domains formed by the alkyl chains and the hydrophilic domains formed by the imidazolium cation and the X− anion. The thermodynamic parameters (ΔGθm, ΔHθm, ΔSθm) for the aggregates formation were derived from the temperature and concentration dependence of conductivity. Thermodynamic potential results indicate that the aggregation of PILs in an organic solvent is spontaneous and driven by entropy change associated with a hydrophobic effect. Moreover, the contribution of the alkyl chains and solvent molecules results in a positive ΔSθm for poly[CnVIm+][Br−] aggregation, which is caused by the destruction of “iceberg clusters”. The contribution of nonsolvent to the change of Gibbs free energy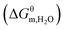 as well as CAC decreases linearly with the addition of nonsolvent in PIL n-PrOH solution, implying that nonsolvent favors PIL aggregation. The results in this work are of great importance for understanding the aggregation process of PIL in an organic solvent and developing PILs-based functional materials with well-ordered structures.
as well as CAC decreases linearly with the addition of nonsolvent in PIL n-PrOH solution, implying that nonsolvent favors PIL aggregation. The results in this work are of great importance for understanding the aggregation process of PIL in an organic solvent and developing PILs-based functional materials with well-ordered structures.
Acknowledgements
This study was supported by the Natural Science Foundation of Zhejiang Province (Grant No. LY13B040004 and LY13B030008). The authors declare that there is no conflict of interest.Notes and references
- J. Dupont, R. F. de Souza and P. A. Z. Suarez, Chem. Rev., 2002, 102, 3667 CrossRef CAS PubMed.
- S. Zhang, Q. Zhang, Y. Zhang, Z. Chen, M. Watanabe and Y. Deng, Prog. Mater. Sci., 2016, 77, 80 CrossRef CAS.
- J. Bowers, C. P. Butts, P. J. Martin, M. C. Vergara-Gutierrez and R. K. Heenan, Langmuir, 2004, 20, 2191 CrossRef CAS PubMed.
- B. Dong, N. Li, L. Zheng, L. Yu and T. Inoue, Langmuir, 2007, 23, 4178 CrossRef CAS PubMed.
- B. Dong, X. Zhao, L. Zheng, J. Zhang, N. Li and T. Inoue, Colloids Surf., A, 2008, 317, 666 CrossRef CAS.
- J. Wang, H. Wang, S. Zhang, H. Zhang and Y. Zhao, J. Phys. Chem. B, 2007, 111, 6181 CrossRef CAS PubMed.
- C. P. Frizzo, I. M. Gindri, C. R. Bender, A. Z. Tier, M. A. Villetti, D. C. Rodrigues, M. Giovanna and M. A. Martins, Colloids Surf., A, 2015, 468, 285 CrossRef CAS.
- M. Ao and D. Kim, J. Chem. Eng. Data, 2013, 58, 1529 CrossRef CAS.
- I. Rodriguez-Palmeiro, I. Rodriguez-Escontrela, O. Rodriguez, A. Arce and A. Soto, RSC Adv., 2015, 5, 37392 RSC.
- Y. Gu, L. Shi, X. Cheng, F. Lu and L. Zheng, Langmuir, 2013, 29, 6213 CrossRef CAS PubMed.
- S. Chauhan and K. Sharma, J. Chem. Thermodyn., 2014, 71, 205 CrossRef CAS.
- J.-H. Choi, Y. Ye, Y. A. Elabd and K. I. Winey, Macromolecules, 2013, 46, 5290 CrossRef CAS.
- J. Tang, W. Sun, H. Tang, M. Radosz and Y. Shen, Macromolecules, 2005, 38, 2037 CrossRef CAS.
- J. Tang, H. Tang, W. Sun, H. Plancher, M. Radosz and Y. Shen, Chem. Commun., 2005, 26, 3325 Search PubMed.
- T. Y. Kim, H. W. Lee, M. Stoller, D. R. Dreyer, C. W. Bielawski, R. S. Ruoff and K. S. Suh, ACS Nano, 2011, 5, 436 CrossRef CAS PubMed.
- J. Huang, C. Tao, Q. An, W. Zhang, Y. Wu, X. Li, D. Shen and G. Li, Chem. Commun., 2010, 46, 967 RSC.
- Q. Zhao, J. W. C. Dunlop, X. Qiu, F. Huang, Z. Zhang, J. Heyda, J. Dzubiella, M. Antonietti and J. Yuan, Nat. Commun., 2014, 5, 4293 Search PubMed.
- W. J. Horne, M. A. Andrews, K. L. Terrill, S. S. Hayward, J. Marshall, K. A. Belmore, M. S. Shannon and J. E. Bara, ACS Appl. Mater. Interfaces, 2015, 7, 8979 CAS.
- Q. Zhao, P. Zhang, M. Antonietti and J. Yuan, J. Am. Chem. Soc., 2012, 134, 11852 CrossRef CAS PubMed.
- J. Yuan and M. Antonietti, Macromolecules, 2011, 44, 744 CrossRef CAS.
- J. Yuan, S. Soll, M. Drechsler, A. H. E. Müller and M. Antonietti, J. Am. Chem. Soc., 2011, 133, 17556 CrossRef CAS PubMed.
- X. Ma, M. Ashaduzzaman, M. Kunitake, R. Crombez, J. Texter, L. Slater and T. Mourey, Langmuir, 2011, 27, 7148 CrossRef CAS PubMed.
- H. He, M. Zhong, B. Adzima, D. Luebke, H. Nulwala and K. Matyjaszewski, J. Am. Chem. Soc., 2013, 135, 4227 CrossRef CAS PubMed.
- R. Marcilla, J. A. Blazquez, J. Rodriguez, J. A. Pomposo and D. Mecerreyes, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 208 CrossRef CAS.
- M. Koebe, M. Drechsler, J. Weber and J. Yuan, Macromol. Rapid Commun., 2012, 33, 646 CrossRef CAS PubMed.
- H. Fan and Z. Jin, Soft Matter, 2014, 10, 2848 RSC.
- R. Zana, J. Colloid Interface Sci., 1980, 78, 330 CrossRef CAS.
- A. Cornellas, L. Perez, F. Comelles, I. Ribosa, A. Manresa and M. T. Garcia, J. Colloid Interface Sci., 2011, 355, 164 CrossRef CAS PubMed.
- A. P. Santos and Y. Levin, Langmuir, 2012, 28, 1304 CrossRef PubMed.
- T. Inoue, H. Ebina, B. Dong and L. Zheng, J. Colloid Interface Sci., 2007, 314, 236 CrossRef CAS PubMed.
- Kabir-ud-Din, P. A. Koya and Z. A. Khan, J. Colloid Interface Sci., 2010, 342, 340 CrossRef CAS PubMed.
- L. Shi, N. Li and L. Zheng, J. Phys. Chem. C, 2011, 115, 18295 CAS.
- B. Sarkar, S. Lam and P. Alexandridis, Langmuir, 2010, 26, 10532 CrossRef CAS PubMed.
- K. Shinoda, M. Kobayashi and N. Yamaguchi, J. Phys. Chem., 1987, 91, 5292 CrossRef CAS.
- Y. Kadam, K. Singh, D. G. Marangoni, J. H. Ma, V. K. Aswal and P. Bahadur, Colloids Surf., A, 2010, 369, 121 CrossRef CAS.
- A. Khan and M. Siddiq, J. Appl. Polym. Sci., 2010, 118, 3324 CrossRef CAS.
- B. Sarkar, V. Ravi and P. Alexandridis, J. Colloid Interface Sci., 2013, 390, 137 CrossRef CAS PubMed.
- S. S. Soni, S. H. Panjabi and N. V. Sastry, Colloids Surf., A, 2011, 377, 205 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: PILs characterization, conductivity data, and the thermodynamic parameters as well as CAC data for poly[C16VIm+][Br−] in n-PrOH/acetone mixed solvents are included. See DOI: 10.1039/c6ra16416a |
| This journal is © The Royal Society of Chemistry 2016 |