Lipid production by Yarrowia lipolytica grown on biodiesel-derived crude glycerol: optimization of growth parameters and their effects on the fermentation efficiency
Abstract
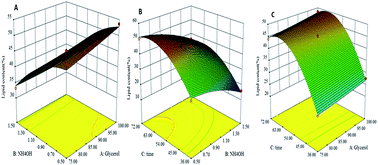
Yarrowia lipolytica, a well-known oleaginous strain for single cell oil (SCO) production was grown in nitrogen-limited flask cultures. The effect of increasing the initial crude glycerol and nitrogen concentration was studied along the fermentation process. Significant biomass and SCO production was reported with a high initial glycerol concentration of 89 g L−1 and 0.54 g NH4OH per L during 6 h. Optimized culture conditions were tested using a 5 L fermenter during two-stage cultivation with a dissolved oxygen shift from 60% to 30% of dissolved oxygen corresponding to 50–80 h−1. Lipid concentration of 13.6 ± 0.8 g L−1 and lipid content 52.7 ± 1.2% (w/w of dry biomass) was obtained which is higher compared with literature values for Yarrowia species grown on crude glycerol based media. The yeast lipids contained mainly oleic, palmitic, linoleic and stearic acids which could serve as perfect precursors for the synthesis of biodiesel.

 Please wait while we load your content...
Please wait while we load your content...