Control of SrO buffer-layer formation on Si(001) using the pulsed-laser deposition technique†
Abstract
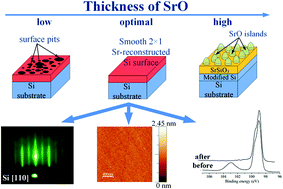
The deoxidation and passivation of a silicon surface represents one of the most important steps in the successful integration of functional oxides with silicon. Due to its reactivity and dissimilar properties with respect to oxides, silicon surfaces are conditioned using various buffer systems. Despite the quality of the resulting surface, these Sr-based buffers have not been commercialized because of the reactivity of the metallic Sr. SrO has demonstrated properties that are competitive with metallic Sr, but a successful integration with silicon has not yet been proven. In the present study we have determined the optimal pulsed-laser deposition (PLD) conditions for the SrO-induced deoxidation of a silicon surface, which results in a 2 × 1 reconstructed surface. Additionally, the as-prepared surface is oxide-free and atomically flat. The results show that the amount of SrO plays the most critical role in the optimization of the whole process. Deposited in batch mode, the amount of SrO affects the morphologies of the surfaces, which change from a dimerized surface to SrO islands and a polycrystalline layer in the final stage. However, in the case of an insufficient amount of deposited SrO, pits are formed on the surface, drastically increasing its roughness. The successful optimization of the PLD conditions for the formation of a SrO buffer layer opens a new pathway for interfacing oxides with silicon.


 Please wait while we load your content...
Please wait while we load your content...