Hydrogen migration in hypoelectronic biicosahedral metallaborane structures†
Amr A. A. Attiaab,
Alexandru Lupan*a and
R. Bruce King*c
aDepartment of Chemistry, Faculty of Chemistry and Chemical Engineering, Babeş-Bolyai University, Cluj-Napoca, Romania. E-mail: alupan@chem.ubbcluj.ro
bNational Institute for Research and Development of Isotopic and Molecular Technologies, Cluj-Napoca, Romania
cDepartment of Chemistry and Center for Computational Quantum Chemistry, University of Georgia, Athens, Georgia 30602, USA. E-mail: rbking@chem.uga.edu
First published on 16th August 2016
Abstract
The biicosahedral metallaboranes CpMB20H17 (M = Pd, Pt; Ru, Os; Mo, W) and CpM′CB19H17 (M′ = Rh, Ir; Re; Ta) have been investigated by density functional theory to supplement the earlier work on the first-row transition metal derivatives CpMB20H17 (M = Ni, Fe) and CpCoCB19H17. The CpMB20H17 (M = Ni, Pd, Pt) and CpMCB19H17 (M = Co, Rh, Ir) systems have the ideal 46 skeletal electrons by the Jemmis rules. Their lowest energy structures have the metal atom in the meta position of the biicosahedron. Hydrogen migration occurs in the low-energy structures of the hypoelectronic systems CpMB20H17 (M = Fe, Ru, Os; Mo, W) to give one or two M–B biicosahedral edges bridged by hydrogen atoms. In CpReCB19H17 and one of the CpTaCB19H17 structures more extensive hydrogen migration occurs to give low-energy structures having terminal M–H bonds and metal vertices of a degree of at least 6. The CpTaCB19H17 system also has a low-energy structure in which the TaCB19 biicosahedron becomes distorted enough to provide a degree 8 vertex for the tantalum atom.
1. Introduction
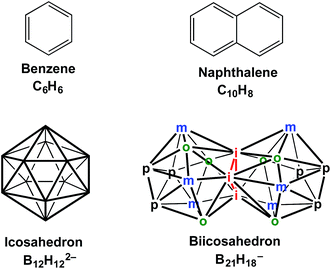
The great stability and relatively low chemical reactivity of the icosahedral borane dianion1,2 B12H122− and the isoelectronic carboranes CB11H12− and C2B10H12 suggest the boron icosahedron to be the three-dimensional analogue of the benzene hexagon (Fig. 1). Structures constructed from the edge-sharing fusion of benzene hexagons lead to the extensive and well-known series of very stable polycyclic aromatic hydrocarbons. However, the analogous chemistry of the three-dimensional borane analogues of the polycyclic aromatic hydrocarbons constructed by the fusion of boron icosahedra is much less developed. Such chemistry is potentially a far richer area than that of polycyclic aromatic hydrocarbons since both edge-sharing and face-sharing of boron icosahedra building blocks are feasible. However, even the simplest biicosahedral boron anion B21H18−, which formally is a three-dimensional analogue of the simplest polycyclic benzenoid hydrocarbon naphthalene (Fig. 1), was synthesized only relatively recently (2007) by Bernhardt, Brauer, Finze, and Willner.3 The carborane CB20H18, which would be an isoelectronic neutral analogue of B21H18− analogous to the three C2B10H12 isomers as isoelectronic neutral analogues of B12H122−, remains unknown. However, the relative thermodynamic stability of the CB20H18 isomers has recently been examined by Vidya and Jemmis4 using density functional theory as extension of previous theoretical studies by this research group on other condensed polyhedral borane derivatives.5,6The stability of monocyclic planar hydrocarbons relates to the number of π-electrons as was first recognized by Hückel7 in the stability of the aromatic sextet in benzene as well as its isoelectronic ionic derivatives C5H5− and C7H7+. Thus planar hydrocarbons and isoelectronic heterocycles exhibit unusual stability as aromatic systems if they contain 4n + 2 skeletal electrons where n is an integer. In an analogous matter for three-dimensional aromatic systems, as reflected most clearly in graph-theoretical approaches to aromaticity,8,9 the number of skeletal electrons can be related to stability and aromaticity by the so-called Wade–Mingos rules.10–12 Thus, for the most spherical deltahedra with n vertices, including the icosahedron for which n = 12, the closed shell structures corresponding to the most stable aromatic species have 2n + 2 skeletal electrons. For example, the icosahedral B12H122− dianion has 26 skeletal electrons with 24 of these skeletal electrons coming from the 12 BH vertices and the final two skeletal electrons from reduction to the dianion.
Jemmis and co-workers13 have shown that the Wade–Mingos rules can be extended to borane cage structures consisting of p fused polyhedra, where 2n + 2p is the favorable number of skeletal electrons. Thus the biicosahedral anion B21H18− requires 2(21 + 2) = 46 skeletal electrons. In the biicosahedral B21H18− structure the 18 boron atoms with external hydrogen atoms unique to one of the two icosahedra each contribute two skeletal electrons. However, the three boron atoms shared by the two icosahedra and thus lacking external hydrogen atoms each contribute all three of their valence electrons as skeletal electrons. Adding the electron provided by reduction of neutral B21H18 to the B21H18− anion leads to the 46 skeletal electrons required by the Jemmis rules consistent with B21H18− being a stable species.
The chemistry of metallaboranes derived from polyhedral boranes by replacement of one or more boron vertices with transition metal moieties is a rich area of chemistry, which was first developed by Hawthorne,14 Grimes,15 and their coworkers. Cyclopentadienylmetal vertices of the type CpM (Cp = η5-C5H5) and Cp*M (Cp* = η5-Me5C5) are particularly useful since the tightly bonded Cp or Cp* ligand blocks extra metal coordination positions and survives rather robust reaction conditions. In this connection each CpM (M = Co, Rh, Ir) vertex of the group 9 metals is a donor of two skeletal electrons similar to a BH vertex. Similarly, each CpM′ (M′ = Ni, Pd, Pt) vertex of the group 10 metals is a donor of three skeletal electrons similar to a CH vertex. Although the chemistry of icosahedral metallaboranes and isoelectronic metallacarboranes, particularly those with CpCo vertices, has been developed extensively during the past half-century, biicosahedral metallaboranes of the type [M]B20H17 ([M] = a transition metal with associated external ligands, commonly Cp) remain unknown.
We recently reported16 a density functional theory study of biicosahedral metallaboranes including CpNiB20H17 and CpCoCB19H17 isoelectronic with B21H18− and CB20H18 with the 46 skeletal electrons required by the Jemmis rules.13 Both CpNiB20H17 and CpCoCB19H17 were found to have perfect biicosahedral structures (Fig. 1) with all 17 hydrogens as external atoms bonded only to a single boron (or carbon) atom. The hypoelectronic iron derivative CpFeB20H17 having only 44 skeletal electrons was found to retain the biicosahedral structure. However, in the lowest energy CpFeB20H17 structure, one of the 17 originally terminal hydrogen atoms on boron migrates towards the iron atom leading to a bridge across one of the Fe–B edges of the biicosahedron.
This interesting example of hydrogen migration to give a hydrogen bridge across one of the Fe–B edges in the hypoelectronic CpFeB20H17 biicosahedron suggested the study of even more highly hypoelectronic systems, notably CpMB20H17 (M = Mo, W) with only 42 skeletal electrons. However, we avoided the first row transition metals vanadium, chromium, and manganese in these studies because of the anticipated complication of low-energy higher spin state structures. In addition, we avoided CpMB20H17 systems with an odd number of electrons, thus limiting this study to singlet spin state structures. Instead, for the odd-numbered transition metals we studied the metallacarbaboranes CpMCB19H17 (M = Ta, Re, Rh, Ir). This study resulted in the discovery of additional examples of hypoelectronic CpMB20H17 and CpMCB19H17 structures with hydrogen atoms bridging M–B edges. Furthermore, for the lowest energy CpReCB19H17 structures, one of the B–H hydrogen atoms was found to migrate completely to the rhenium atom.
2. Theoretical methods
Full geometry optimizations were carried out on the CpMB20H17 (M = Pd, Pt, Ru, Os, Mo, W) and CpMCB19H17 (M = Rh, Ir, Re, Ta) systems by using the B3LYP DFT functional17–20 coupled with the SDD (Stuttgart/Dresden ECPs plus DZ)21 basis set for the metal ions and the double zeta 6-31G(d) basis set for the remaining atoms. The lowest energy structures were then reoptimized using the M06-L DFT functional22 combined with the SDD basis set for the metal ions and the triple zeta 6-311G(d,p) basis set for the remaining atoms. The latter data are discussed in detail in this paper. The initial CpMB20H17 structures were chosen by substituting a CpM vertex for a BH vertex at all non-equivalent positions in a biicosahedral B21H18 unit (Fig. 1). The initial CpMCB19H17 structures were obtained by first substituting a boron vertex with a carbon vertex in all possible positions of the initial B21H18 biicosahedron followed by introduction of a CpM vertex in all of the remaining available positions. The stationary points after optimization were checked by calculations of the harmonic vibrational frequencies. If significant imaginary frequencies were found, the optimization was continued by following the normal modes corresponding to imaginary frequencies to insure that genuine minima were obtained. Except as noted, all of the reported low-energy optimized CpMB20H17 and CpMCB19H17 structures have HOMO–LUMO gaps of at least 2.0 eV. All calculations were performed using the Gaussian 09 package23 with the default settings for the SCF cycles and geometry optimization (see the ESI†).The CpMB20H17 structures are designated as B20M-x where M is the transition metal atom and x is the relative order of the structure on the potential energy scale. The CpMCB19H17 structures are analogously designated as B19CM-x. The lowest energy optimized structures depicted in Fig. 2–7 are those with the third row transition metals from tantalum to platinum. Further details on these structures are given in the ESI.†
3. Results and discussion
3.1 The CpMB20H17 systems
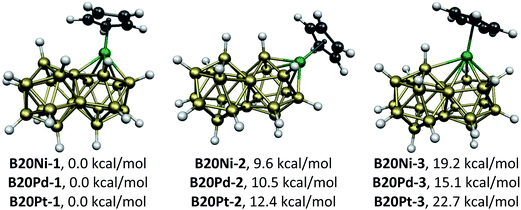
The CpMB20H17 (M = Ni, Pd, Pt) systems of the group 10 metals (Fig. 2) have the optimum 46 skeletal electrons for a biicosahedral structure. For all three metals the lowest energy structures B20M-1 have the metal atom located at a meta vertex of the biicosahedron. The next higher energy CpMB20H17 structures B20M-2, lying 9.6 (Ni), 10.5 (Pd), and 12.4 (Pt) kcal mol−1 above B20M-1, have the metal atom located at a para vertex of the icosahedron. In both of these structures the B20H17 unit derived from the biicosahedron functions as a pentahapto ligand. The highest energy CpMB20H17 structures B20M-3 (M = Ni, Pd, Pt), lying 19.2 (Ni), 15.1 (Pd), and 22.7 (Pt) kcal mol−1 in energy above B20M-1, have the metal atoms located at an ortho vertex of the biicosahedron. In this case the B20H17 unit derived from the biicosahedron functions as a hexahapto ligand.The CpMB20H17 (M = Fe, Ru, Os) systems of the group 8 metals (Fig. 3) are hypoelectronic systems having only 44 rather than the ideal 46 skeletal electrons for their biicosahedral structures. This deficiency of two skeletal electrons is balanced by migration of one of the terminal hydrogens on a boron atom adjacent to the metal atom towards the metal atom to bridge one of the M–B biicosahedral edges. This bridging process brings one of the otherwise non-bonding electron pairs on the metal atom into the skeletal bonding thereby making them effectively 46 skeletal electron systems like the Group 10 CpMB20H17 (M = Ni, Pd, Pt) derivatives without any bridging hydrogen atoms. For the bridging hydrogen atoms in the CpMB20H17 (M = Ru, Os) structures the M–H distances range from 1.73 to 1.79 Å and the B–H distances range from 1.32 to 1.36 Å.
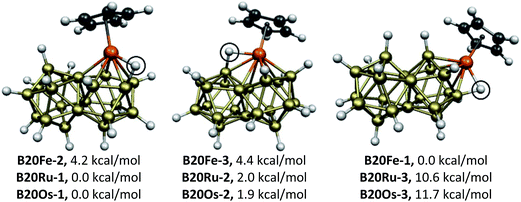 | ||
| Fig. 3 The three optimized CpMB20H17 (M = Fe, Ru, Os) structures. The bridging hydrogen atoms are circled. | ||
The relative energy ordering of the isomeric CpMB20H17 structures of the second and third row Group 8 metals ruthenium and osmium is different from that of the previously studied iron derivative CpFeB20H17 (Fig. 3). Thus the lowest energy CpMB20H17 (M = Ru, Os) structure has the metal atom in a meta position of the biicosahedron with a hydrogen atom bridging an edge from the metal atom to an adjacent boron atom in a para position of the biicosahedron. However, the lowest energy CpFeB20H17 structure has the metal atom in a para position. The corresponding CpMB20H17 (M = Ru, Os) structures lie ∼11 kcal mol−1 in energy above their lowest energy isomers. For CpMB20H17 (M = Ru, Os) the structures B20M-2 (M = Ru, Os) with the metal in an ortho position of the biicosahedron lie only ∼2 kcal mol−1 in energy above the corresponding B20M-1 structures.
The still more hypoelectronic CpMB20H17 (M = Mo, W) derivatives have only 42 skeletal electrons and thus lack 4 skeletal electrons for the ideal 46 skeletal electrons for the biicosahedron. The lowest energy such structures by more than 8 (Mo) and 10 (W) kcal mol−1 have the B20H17 unit functioning as a hexahapto ligand to the metal atom located in an ortho position of the biicosahedron (Fig. 4). Five of the six M-B edges in the B20M-1 (M = Mo, W) structures are part of the icosahedron containing the metal atom whereas the sixth M–B edge goes to the nearest boron atom of the other icosahedron. The hydrogen atom on the latter boron atom migrates towards the metal atom to bridge this M–B edge with M–H distances of ∼1.86 Å and B–H distances of ∼1.26 Å. The B20M-1 (M = Mo, W) structures have relatively low HOMO–LUMO gaps of ∼1.0 eV compared with most of the other CpMB20H17 structures, which have HOMO–LUMO gaps of 2.0 eV or higher.
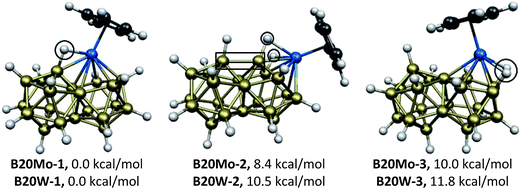 | ||
| Fig. 4 The three optimized CpMB20H17 (M = Mo, W) structures. The bridging hydrogen atoms and the unusually short M–B intericosahedral distances are enclosed. | ||
The next CpMB20H17 (M = Mo, W) structures B20M-2, lying 8.4 (Mo) and 10.5 (W) kcal mol−1 in energy above B20M-1, have the metal atom in a para position of the biicosahedron with two hydrogen bridges from this metal atom to boron atoms located at adjacent meta positions (Fig. 4). Another interesting feature of the B20M-2 structures are unusually short bonding intericosahedral ortho–ortho B–B distances of ∼1.93 Å (in a box in Fig. 4) as compared with the usual non-bonding ortho–ortho B⋯B distances of 2.2 to 2.3 Å in the other structures.
The third CpMB20H17 (M = Mo, W) structures B20M-3, lying 10.0 (Mo) and 11.8 (W) kcal mol−1 in energy above B20M-1 and thus only slightly higher energetically than B20M-2, also have two M–B edges bridged by hydrogen atoms (Fig. 4). However, in B20M-3 the metal atoms are located in a meta position and the intericosahedral ortho–ortho B–B distances are a normal non-bonding ∼2.27 Å.
The B20H17 ligand in the CpMB20H17 complexes can be considered as a nido trianion B20H173− with the favored 46 skeletal electrons for a structure derived from a biicosahedral system. Therefore, the metal atoms in the CpMB20H17 complexes are in the formal M(IV) oxidation state regardless of whether the bonding of the B20H17 ligand to the metal involves hydrogen atoms bridging B–M edges of the resulting deltahedron. The metal atoms in the Group 10 CpMB20H17 complexes (M = Ni, Pd, Pt) without bridging hydrogen atoms (Fig. 2) thus have the d6 electronic configuration similar to extensive series of octahedral complexes of Pt(IV), Co(III), Fe(II), etc. In this sense the B20H173− ligand stabilizes high formal oxidation states.
3.2 The CpMCB19H17 systems
The CpMCB19H17 systems studied for the transition metals of odd atomic number (Rh, Ir, Re, and Ta) are more complicated than the CpMB20H17 systems because of the large number of isomers. Thus for each location of the metal atom on the biicosahedral skeleton there are several possible locations for the carbon vertex.The metallacarbaboranes CpMCB19H17 (M = Co, Rh, Ir) are isoelectronic with CpM′B20H17 (M′ = Ni, Pd, Pt) and thus have the ideal 46 skeletal electrons for a biicosahedral structure. The lowest energy CpMCB19H17 structures are seven isomers with the metal atom in a meta position of the biicosahedron (Fig. 5). The energy orderings of these seven isomers are similar for the three metals except for the isomer with an M–C edge to the carbon atom located at an adjacent para vertex. The relative energies of this particular isomer increase in going from cobalt to rhodium and then iridium at 1.5, 5.3, and 8.5 kcal mol−1, respectively, above the lowest energy isomers B19CM-1 (M = Co, Rh, Ir).
 | ||
| Fig. 5 The seven optimized CpMCB19H17 (M = Co, Rh, Ir) structures within 13 (Co), 10 (Rh), and 15 (Ir) kcal mol−1 of energy of the global minima M19CM-1. | ||
The rhenium system CpReCB19H17, like the isoelectronic CpMB20H17 (M = Fe, Ru, Os) systems, has only 44 apparent skeletal electrons and thus lacks two electrons for the ideal biicosahedral 46 skeletal electrons. The three lowest energy CpReCB19H17 structures all have the rhenium atom in an ortho position of the biicosahedron, which functions as a hexahapto ligand (Fig. 6). The hydrogen atom on the boron atom of the opposite icosahedron bonded to the rhenium atom has migrated completely to the rhenium atom leading to a terminal Re–H bond. Thus the rhenium atom in the three lowest energy CpReCB19H17 structures has effectively inserted into the B–H bond that indirectly provides the two extra electrons to convert the 44 apparent skeletal electron CpReCB19H17 system into an effective 46 skeletal electron CpRe(H)CB19H16 system. In these three CpRe(H)CB19H16 structures, which differ only in the location of carbon relative to rhenium, the CpRe(H) vertex is a donor of one skeletal electron and all four boron atoms lacking external hydrogen atoms are donors of three skeletal electrons.
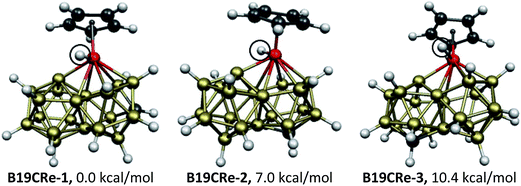 | ||
| Fig. 6 The three optimized CpReCB19H17 structures within 19 kcal mol−1 of B19CRe-1. The terminal hydrogen atoms bonded to rhenium are circled. | ||
The tantalum system CpTaCB19H17, like the isoelectronic CpMB20H17 (M = Mo, W) systems, has only 42 apparent skeletal electrons and thereby is four electrons short of the ideal 46 skeletal electrons for a biicosahedral borane. Thus the CpTaCB19H17 system is isoelectronic with the CpMB20H17 (M = Mo, W) systems. The four extra electrons needed for CpTaCB19H17 to acquire the favorable 46 effective skeletal electrons arise indirectly through combinations of bridging hydrogen atoms, hydrogen migration from boron to tantalum, and tantalum vertex degrees of seven or even eight. Such high tantalum vertex degrees allow tantalum to contribute more than the usual three valence orbitals to the skeletal bonding framework. This effectively brings otherwise external tantalum lone pairs into the skeletal bonding. Thus a CpTa vertex providing the usual three internal orbitals for skeletal bonding is a −2 skeletal electron donor, i.e. an acceptor or two skeletal electrons. However, a CpTa vertex providing five internal orbitals for skeletal bonding analogous to that proposed for the CpRe vertices in the experimentally known oblatocloso dirhenaboranes,24 Cp2*Re2Bn−2Hn−2, becomes a donor of two skeletal electrons.
The lowest energy CpTaCB19H17 structure B19CTa-1 as well as B19CTa-4, lying 7.5 kcal mol−1 in energy above B19CTa-1, has one Ta–B bond bridged by a hydrogen atom and a degree 7 tantalum vertex (Fig. 7). The bridging hydrogen can bring one of the otherwise external tantalum lone pairs into the skeletal bonding. In addition, if the degree 7 tantalum vertex provides four rather than the usual three internal orbitals to the skeletal bonding, another external tantalum lone pair is brought into the skeletal bonding. In this way the CpTaCB19H17 structures B19CTa-1 and B19CTa-4 can have effectively the optimum 46 skeletal electrons for a biicosahedral structure. Structures B19CTa-1 and B19CTa-4 differ only in the location of the carbon atom in the biicosahedral framework. However, in both structures the tantalum atom is within bonding distance of the carbon atom.
 | ||
| Fig. 7 The four optimized CpTaCB19H17 structures within 10 kcal mol−1 of B19CTa-1. Hydrogen atoms bridging Ta–B edges and terminal hydrogen atoms bonded to tantaum are encircled. | ||
The CpTaCB19H17 structure B19CTa-2, lying only 2.2 kcal mol−1 in energy above B19CTa-1, is interesting since it has a degree 8 tantalum vertex but no bridging hydrogen atoms (Fig. 7). The 8-vertex B7C subunit of the biicosahedron to which the tantalum is bonded resembles the bent octahapto pentalene ligand in bis(pentalene)titanium, (η8-C8H6)2Ti, and related complexes.25 If the CpTa vertex provides five internal orbitals for the skeletal bonding, then B19CTa-2 has the 46 skeletal electrons for a biicosahedral structure. However, bonding of the tantalum atom to eight atoms in the biicosahedron leads to considerable distortion.
The remaining low-energy CpTaCB19H17 structure B19CTa-3, lying 7.3 kcal mol−1 in energy above B19CTa-1, resembles the three CpReCB19H17 structures (Fig. 6) since a hydrogen has migrated completely from boron to the metal atom and the CB19H17 unit functions as a hexahapto ligand (Fig. 7). Since none of the hydrogens in B19CTa-3 bridge Ta–B edges, this structure appears to have 44 skeletal electrons rather than the ideal 46 skeletal electrons for a biicosahedron.
The CB19H17 ligand in the CpMCB19H17 complexes can be considered to be a dianion CB19H172− with 46 skeletal electrons isoelectronic with the B20H173− trianion discussed above. Thus the metal atoms in the CpMCB19H17 complexes with intact CB19H17 ligands are in the formal M(III) oxidation state regardless of whether the bonding of the CB19H17 ligand to the metal atom involves hydrogen bridging across B–M edges. The CpMCB19H17 complexes (M = Co, Rh, Ir) are then related to the numerous octahedral M(III) complexes of these d6 metals. The CB19H16 ligand found in the CpM(H)CB19H16 structures with a separate hydride ligand bonded to the metal, including the three lowest energy CpReCB19H17 structures, can be derived by deprotonation of CB19H17 and thus is the trianion CB19H173−. Considering the Re–H hydrogen in the CpRe(H)CB19H16 structures as the hydride anion places the rhenium atom in the formal Re(V) oxidation state after considering the Cp− ligand as a monoanion. This is a reasonable oxidation state for rhenium considering such stable species as ReCl5. A similar analysis assigns the particularly favorable formal d0 Ta(V) oxidation state to the CpTa(H)CB19H16 species B19CTa-3 (Fig. 7). In the CpTaCB19H17 complexes without a terminal Ta–H hydrogen, such as B19CTa-1, B19CTa-2, and B19CTa-4, the degree 7 or 8 tantalum vertices suggest an arachno CB19H17 ligand with two open faces corresponding to a CB19H174− tetraanion. The combination of a tetraanionic CB19H174− ligand and a monoanionic Cp− ligand gives the tantalum atom in all three complexes B19CTa-1, B19CTa-2 and B19CTa-4 the favorable d0 Ta(V) formal oxidation state.
4. Conclusions
The group 10 metal biicosahedral metallaboranes CpMB20H17 (M = Ni, Pd, Pt) as well as the isoelectronic group 9 metal biicosahedral metallacarbaboranes CpM′CB19H17 (M′ = Co, Rh, Ir) have the ideal 46 skeletal electrons suggested by the Jemmis rules for fused polyhedral boranes based on the earlier Wade–Mingos rules for polyhedral boranes. The 17 hydrogen atoms in these structures are all terminal hydrogen atoms. The lowest energy structures have the metal atom located at a meta position of the biicosahedron.The group 8 metal biicosahedral metallaboranes CpMB20H17 (M = Fe, Ru, Os) are hypoelectronic since they have only 44 skeletal electrons. This electron deficiency relative to the ideal 46 skeletal electrons results in migration of a B–H hydrogen towards the metal atom. The result is a hydrogen atom bridging an M–B edge of the biicosahedron in the lowest energy structures. The three possible such CpFeB20H17 structures with the iron atom in the ortho, meta, and para positions of the biicosahedron (Fig. 1) lie within ∼5 kcal mol−1 of energy. However, for CpMB20H17 (M = Ru, Os) the meta isomers have the lowest energies. More extensive B–H migration occurs in the biicosahedral rhenaboranes CpReCB19H17 valence isoelectronic with CpMB20H17 (M = Ru, Os) leading to structures with a terminal hydrogen bonded to the rhenium atom. As a result of such B–H migration, the three lowest energy CpReCB19H17 structures all have the rhenium atom in the ortho position of the biicosahedron and differ only in the location of the vertex carbon atom.
The group 6 metal biicosahedral metallaboranes CpMB20H17 (M = Mo, W) are even more hypoelectronic with only 42 skeletal electrons relative to the ideal 46 skeletal electrons. The lowest energy CpMB20H17 (M = Mo, W) structures have the metal atom in an ortho position of the biicosahedron bonded to six boron atoms with one of these M–B edges bridged by a hydrogen atom. The next two CpMB20H17 structures in terms of energy, lying ∼10 kcal mol−1 above the lowest energy structures, have the metal atom in para and meta positions of the biicosahedron. Two of the M–B edges in these structures are bridged by hydrogen atoms to compensate for the hypoelectronicity.
The biicosahedron in the lowest energy structure of the tantalum system CpTaCB19H17 isoelectronic with CpMB20H17 (M = Mo, W) is distorted to provide a degree 7 vertex for the tantalum atom as well as a hydrogen bridging one of the Ta–B edges. Lying only ∼2 kcal mol−1 above this CpTaCB19H17 structure is an isomeric structure with an even more highly distorted biicosahedron to provide a degree 8 vertex for the tantalum atom. This CpTaCB19H17 structure has exclusively terminal hydrogen atoms. Another low-energy CpTaCB19H17 structure exhibits migration of a B–H hydrogen to become a terminal hydrogen bonded to the tantalum.
In summary, the biicosahedral CpMB20H17 (M = Ni, Pd, Pt) and CpMCB19H17 (M = Co, Rh, Ir) systems have the 46 skeletal electrons favored by the Jemmis rules.13 Boron–hydrogen migration occurs in the low-energy structures of the hypoelectronic systems CpMB20H17 (M = Fe, Ru, Os; Mo, W) to give structures having one or two M–B biicosahedral edges bridged by hydrogen atoms. For CpReCB19H17 and one of the CpTaCB19H17 structures, migration of the B–H hydrogen occurs to an even larger extent to give low-energy structures having terminal M–H bonds. The CpTaCB19H17 system also has a low-energy structure in which the TaCB19 biicosahedron becomes distorted enough to provide a degree 8 vertex for the tantalum atom.
Acknowledgements
Funding from the Romanian Ministry of Education and Research, (Grant PN-II-RU-TE-2014-4-1197) is gratefully acknowledged. Additional computational resources were provided by the high-performance computational facility MADECIP, POSCCE, COD SMIS 48801/1862 co-financed by the European Regional Development Fund of the European Union.References
- Boron Hydride Chemistry, ed. E. L. Muetterties, Academic Press, New York, 1975 Search PubMed.
- R. N. Grimes, Carboranes, Academic Press, New York, 1970 Search PubMed.
- E. Bernhardt, D. J. Brauer, M. Finze and H. Willner, Angew. Chem., Int. Ed., 2007, 46, 2927 CrossRef CAS PubMed.
- K. Vidya and E. D. Jemmis, J. Organomet. Chem., 2015, 798, 91 CrossRef CAS.
- E. D. Jemmis, B. Pathak and A. Anoop, Inorg. Chem., 2005, 44, 7184 CrossRef CAS PubMed.
- O. Shameema, B. Pathak and E. D. Jemmis, Inorg. Chem., 2008, 47, 4375 CrossRef CAS PubMed.
- E. Hückel, Z. Phys., 1932, 76, 628 CrossRef.
- R. B. King and D. H. Rouvray, J. Am. Chem. Soc., 1977, 99, 7834 CrossRef CAS.
- J.-i. Aihara, J. Am. Chem. Soc., 1978, 100, 3339 CrossRef CAS.
- K. Wade, Chem. Commun., 1971, 792 RSC.
- D. M. P. Mingos, Nature (London), Phys. Sci., 1972, 99, 236 Search PubMed.
- D. M. P. Mingos, Acc. Chem. Res, 1984, 17, 311 CrossRef CAS.
- E. D. Jemmis, M. M. Balakrishnarajan and P. D. Pancharatna, Chem. Rev., 2001, 102, 93–144 CrossRef.
- K. P. Callahan and M. F. Hawthorne, Adv. Organomet. Chem., 1976, 14, 145 CrossRef CAS.
- R. N. Grimes, Acc. Chem. Res, 1983, 16, 22 CrossRef CAS.
- A. A. Attia, A. Lupan and R. B. King, Phys. Chem. Chem. Phys., 2016, 18, 11707 RSC.
- S. H. Vosko, L. Wilk and M. Nusair, Can. J. Phys., 1980, 58, 1200 CrossRef CAS.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS.
- P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 37, 785 CrossRef.
- D. Andrae, U. Haussermann, M. Dolg, H. Stoll and H. Preuss, Theor. Chim. Acta, 1990, 77, 123 CrossRef CAS.
- D. G. Truhlar and Y. Zhao, Theor. Chem. Acc., 2008, 20, 215 Search PubMed.
- Gaussian 09 (Revision E.01),Gaussian, Inc., Wallingford, CT, 2009 Search PubMed, The complete reference is given in the ESI.†.
- R. B. King, Inorg. Chem., 2006, 45, 8211 CrossRef CAS PubMed.
- K. Jonas, P. Kolb, G. Kollbach, B. Gabor, R. Mynott, K. Angermund, O. Heinemann and C. Krüger, Angew. Chem., Int. Ed., 1997, 36, 1714 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Table S1: distance table for the CpTaCB19H17 structures; Table S2: distance table for the CpMoB20H17 structures; Table S3: distance table for the CpWB20H17 structures; Table S4: distance table for the CpReCB19H17 structures; Table S5: distance table for the CpRuB20H17 structures; Table S6: distance table for the CpOsB20H17 structures; Table S7: distance table for the CpRhCB19H17 structures; Table S8: distance table for the CpIrCB19H17 structures; Table S9: distance table for the CpPdB20H17 structures; Table S10: distance table for the CpPtB20H17 structures; Table S11: orbital energies and HOMO/LUMO gaps; Table S12: vibrational frequencies and IR intensities of all structures; Gaussian 09 parameters for SCF cycles and geometry optimizations; complete Gaussian09 reference (ref. 3); Separate concatenated xyz file containing all of the optimized biicosahedral metallaborane structures. See DOI: 10.1039/c6ra16304a |
| This journal is © The Royal Society of Chemistry 2016 |