Comprehensive morphological and structural investigation of cellulose I and II nanocrystals prepared by sulphuric acid hydrolysis†
Abstract
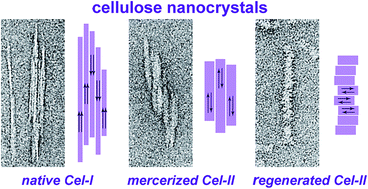
Cellulose nanocrystals (CNCs) were produced from eucalyptus wood pulp using three different methods: (i) classical sulphuric acid hydrolysis (CN-I), (ii) acid hydrolysis of cellulose previously mercerized by alkaline treatment (MCN-II), and (iii) solubilization of cellulose in sulphuric acid and subsequent recrystallization in water (RCN-II). The three types of CNCs exhibited different morphologies and crystalline structures that were characterized using complementary imaging, diffraction and spectroscopic techniques. CN-I corresponded to the type I allomorph of cellulose while MCN-II and RCN-II corresponded to cellulose II. CN-I and MCN-II CNCs were acicular particles composed of a few laterally-bound elementary crystallites. In both cases, the cellulose chains were oriented parallel to the long axis of the particle, although they were parallel in CN-I and antiparallel in MCN-II. RCN-II particles exhibited a slightly tortuous ribbon-like shape and it was shown that the chains lay perpendicular to the particle long axis and parallel to their basal plane. The unique molecular and crystal structure of the RCN-II particles implies that a higher number of reducing chain ends are located at the surface of the particles, which may be important for subsequent chemical modification. While other authors have described nanoparticles prepared by regeneration of short-chain cellulose solutions, no detailed description was proposed in terms of particle morphology, crystal structure and chain orientation. We provide such a description in the present paper.

 Please wait while we load your content...
Please wait while we load your content...