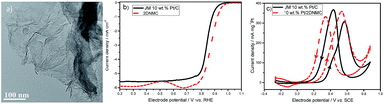
An ultrathin 2D semi-ordered mesoporous silica film: co-operative assembly and application†
Kai Wana,
Zhi-peng Yua,
Quan-bing Liua,
Jin-hua Piaob,
Yu-ying Zhengc and
Zhen-xing Liang*a
aKey Laboratory on Fuel Cell Technology of Guangdong Province, School of Chemistry and Chemical Engineering, South China University of Technology, Guangzhou 510641, P. R. China. E-mail: zliang@scut.edu.cn
bSchool of Food Science and Engineering, South China University of Technology, Guangzhou 510641, P. R. China
cSchool of Chemical Engineering and Light Industry, Guangdong University of Technology, Guangzhou 510006, P. R. China
First published on 5th August 2016
Abstract
A novel ultrathin silica film with semi-ordered fingerprint-like mesopores is synthesized with the aid of the dual templates of graphene oxide (go) and tri-block copolymer P123. The formation is proposed to proceed by a co-operative assembly mechanism. As an application, the silica film is used to synthesize a 2D nitrogen-doped mesoporous carbon film, which yields superior kinetic and diffusion-limit currents than the commercial Pt/C catalyst.
In very recent years, 2D nanostructured materials have attracted enormous attention due to their unique properties and wide applications in catalysis, optoelectronics, drug delivery, and energy-related devices.1–6 Among various strategies, the template method has been widely employed to control the nanostructure and the composition at the atom level.7–10 Graphene, as a 2D substrate, is effective in assembling the 2D nanostructure for both inorganic and organic materials.11–13 For example, Kim14 deposited AuCN nanowires on pristine graphene and aligned them in the zigzag lattice direction. This self-organizing growth process was proposed to be driven by both the lattice matching and the π interaction to gold atoms. However, it is noted that the alignment of the inorganic guest material may not be that easy as the pristine graphene is chemically inert.1 In comparison, graphene oxide (GO), an oxidized derivative of graphene, is enriched with oxygen-containing groups on the surface.15,16 The hydroxyl groups allow GO to form hydrogen bonds with other hydroxyl-rich molecules; the carboxyl groups located at the edges result in negatively charged GO in basic media; and the delocalized electrons over sp2 carbon atoms introduce π–π interaction with other π-conjugated materials.11,17 As such, GO shows an amphiphilic feature, and thus, facilitates the self-assembly of both inorganic and organic guests as a surfactant sheet.13 Feng18 assembled mesoporous silica onto either side of GO to generate sandwich-structured nanocomposites, which was used as a template to synthesize graphene-based carbon. Dabiri19 immobilized ultrasmall Au nanoparticles on such periodic mesoporous silica-graphene sandwich nanocomposites, which yielded an excellent catalytic activity to some typical organic reactions. Ordered mesoporous silicas (OMS), like SBA-15, have attracted much attention due to the unique pore features.20,21 Linear triblock copolymer poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) has been widely used as the template during the synthesis.
In this work, a novel ultrathin silica film with semi-ordered fingerprint-like mesopores is synthesized with the aid of the dual templates of GO and P123. Characterization reveals that the resultant silica features high aspect ratio and specific surface area. To clarify the mechanism, various templates (including GO, reduced graphene oxide (RGO), P123) are used and the effect of the concentration is investigated. By using RGO and P123, the resultant silica shows essentially the same morphology and structure to that with sole P123. In comparison, the introduction of GO yields a considerable change in silica, of which both the morphology and structure evolve with the GO concentration. Increasing the GO concentration gradually lowers the ordering degree of the mesopores, and increases the aspect ratio of the silica block. 2D semi-ordered mesoporous silica film is obtained at a sufficiently high GO concentration. Based on the above findings, the 2D semi-ordered mesoporous silica film is suggested to result from the co-operative assembly of the silica species on the dual templates of GO and P123. To our knowledge, this is the first report to synthesize ultrathin 2D silica film with semi-ordered fingerprint-like parallel mesopores. As an example of application, the silica film then is used as the template to synthesize 2D nitrogen-doped mesoporous carbon films (2DNMC), which are of great interests in electrochemical energy technologies. The 2DNMC film is featured by an ultrathin thickness (1.0 nm), high specific surface area and accessible mesopore surface, enabling itself a good candidate as both the catalyst and support. Electrochemical test confirms that as either the catalyst itself or the support for Pt, the 2DNMC film yields superior kinetic and diffusion-limit currents than does the commercial Pt/C catalyst for the oxygen reduction reaction and methanol oxidation reaction. This finding consolidates that the unique 2D mesoporous structure enables both full utilization of the catalytically active site and facile mass transfer of the active species, which are highly desirable in the electrochemical energy conversion and storage.
Fig. 1 shows the TEM images of the synthesized silica by using various templates. As seen in Fig. 1a and b, with the sole template of P123, SBA-15 shows the same morphology and pore structure with the previous reports.20,22,23 The particles are uniform in size and the ordered mesopores are clearly seen. When RGO is used as a co-template, no significant change can be observed in either the morphology or pore features (Fig. 1c and d). In comparison, by using GO and P123 as the dual templates, the resultant silica turns from the aforementioned 3D particles to 2D films (3 nm in thickness, Fig. S2†) with extremely high aspect ratio, as seen in Fig. 1e. Fingerprint-like parallel mesopores can be clearly seen in Fig. 1f.
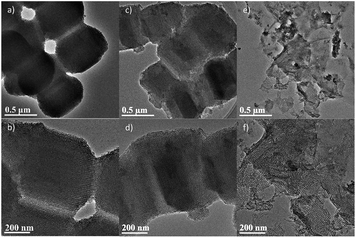 | ||
| Fig. 1 TEM images of the resultant silicas: (a and b) SBA-15; (c and d) SiO2/RGO-5.64; (e and f) SiO2/GO-5.64. | ||
Fig. 2a shows the small angel X-ray diffraction patterns. It is seen that SBA-15 shows three well-resolved reflection peaks at (100), (110) and (200), revealing the ordered porous nature with p6mm hexagonal symmetry. The co-template RGO does not degrade the ordering of the pore structure, which agrees with the TEM results. The three well-resolved peaks indicates that there is no preferential orientation of the unit cell to the RGO surface. In comparison, the reflection peaks cannot be seen in presence of GO at the concentration of 5.64 mg mL−1, indicating degraded ordering of the pore structure.
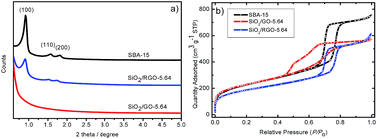 | ||
| Fig. 2 (a) Small angle X-ray diffraction and (b) nitrogen sorption isotherms of the synthesized silicas. | ||
Fig. 2b shows the corresponding nitrogen ad/desorption isotherms. It is seen that both SBA-15 and SiO2/RGO-5.64 show the same shape of the type-IV isotherm with an H1 hysteresis loop, confirming their mesoporous structure.20,23 As such, it is concluded that RGO yields a negligible effect on the assembly of silica oligomers on P123, and RGO cannot be used as a 2D co-template in the synthesis. In comparison, SiO2/GO-5.64 shows a type-IV isotherm but associated with a unique hysteresis loop, confirming that the pore structure is different from the other two samples. The bulge in the desorption branch at P/P0 = 0.50 can be explained by the tensile strength effect.24 The effect occurs when the interconnected pores filled with nitrogen are emptied through smaller pores or narrower sections along the pores.
The effect of the co-template GO is further investigated by changing its concentration from 1.41 to 5.64 mg mL−1. Fig. 3 shows the evolution in the morphology of silica. At the low concentration of 1.41 mg mL−1, the morphology is solid particle with ordered mesoporous pores. The result is pretty similar to that of GO-free SBA-15 (see Fig. 1a and b). When the concentration increases to 2.82 mg mL−1, it is seen that besides solid particles, a fraction of silica at the edge is in the form of thin film (circle-noted). When the concentration reaches 4.23 mg mL−1 (see Fig. 3e and f), solid particles are not seen and the morphology of silica turns to 2D films. In addition, the characteristic dimension of the silica film is found to decrease when the GO concentration increases from 4.23 to 5.64 mg mL−1 (see Fig. 3g and h). At 4.23 mg mL−1, the silica films are large and should originate from the replica of the GO sheet; in comparison, at 5.64 mg mL−1, the silica films are smaller in diameter and basically in fragments. The above findings strongly indicate that GO, as a co-template, triggers a co-operative assembly among the silica precursor and the template P123.
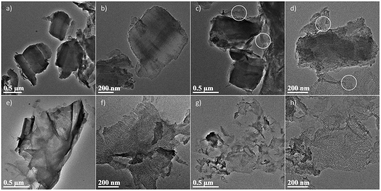 | ||
| Fig. 3 TEM images of the SiO2/GO-x: (a and b) x = 1.41, (c and d) x = 2.82, (e and f) x = 4.23, (g and h) x = 5.64. | ||
The pore ordering is investigated by SA-XRD, as shown in Fig. 4a and S3.† It is seen that SiO2/GO-1.41 shows three reflection peaks at (100), (110) and (200), which are gradually weakened when increasing the GO concentration. This result indicates that the hexagonal packing degree of the mesopores lowers gradually in this process. In this sense, the intensity of the peak can be seen as an indicator of the thickness of the ordered mesoporous silica film. Notably, the two high-indexed peaks decay much faster than the peak (100) with increasing the GO concentration, indicating that the c-axis of the hexagonal unit cell is oriented parallel to the GO surface. This finding confirms the above analysis that GO plays a key role in assembling both the silica precursor and surfactant.
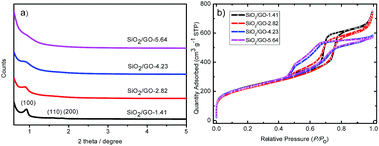 | ||
| Fig. 4 (a) Small angle X-ray diffraction and (b) nitrogen sorption isotherms of SiO2/GO-x (x = 1.41, 2.82, 4.23, 5.64 mg mL−1). | ||
Fig. 4b shows the corresponding nitrogen sorption isotherms. All the four samples basically show the same isotherms, except that the bulge at P/P0 = 0.50 gradually increases with the GO concentration. Table S2† shows that the four samples have similar specific surface area, revealing that the GO concentration has some but not significant effect on the mesopore features. As such, it is concluded that the mesopore features mainly rely on the template of P123, and the 2D morphology depends on the co-template GO.
To further clarify the role of GO, silica was synthesized by using GO as the sole template without adding P123 (see Fig. S4†). The silica is basically in the form of disordered fragment film. It is inferred that the silica precursor is hydrolysed and then assembled onto the GO sheet, which should be driven by both the hydrogen bonding between them. It is noted that the above-mentioned semi-ordered mesoporous structure is not observed in the absence of P123, indicating its key role in generating the mesoporous structure.
In summary, the above findings confirm that P123 and GO act as the dual templates to direct the formation of the semi-ordered mesoporous silica film. It is proposed that this novel nanostructure is formed by a so-called co-operative assembly mechanism. Two critical points are involved in this mechanism: (i) the formation of the mesochannels results from the assembly of the silica precursor onto the P123 aggregates, which has been well documented in literature;20,25,26 (ii) when GO is present, the assembly becomes complicated as the three components (GO, P123, silica oligomer) can interact with each other by the hydrogen bonding. Such a competition depends on the concentration of each component, which yields an evolution in the morphology of silica. At a low GO concentration, the self-assembly of the silica precursor onto the P123 aggregates is predominant, which generates the conventional ordered mesoporous SBA-15 particles. At sufficiently high GO concentrations, the high surface area of GO enables the submonolayer adsorption of cylindrical P123 micelle onto the 2D substrate, which results in the formation of dual templates P123/GO. In the meantime, tetraethyl orthosilicate (TEOS) is adsorbed and hydrolyses at the interface; and thereafter, ultrathin silica film gradually develops.
Fig. 5 shows the schematic model of the co-operative assembly mechanism. First, the hydrophobic segments of P123 molecules tend to aggregate and form spherical micelles.25–28 In the presence of GO, the spherical micelles can interact with the oxygen-containing surface of GO by hydrogen bonding. To further decrease the interfacial energy, the spherical micelles are assembled side-by-side, and thus aligned into bundles. As such, the P123 bundles are adsorbed on the surface of GO to form the dual templates (Fig. 5a and b). Second, the monomeric silica source is hydrolyzed in the acidic solution to form the Si(OMe)4−n(OH2+)n species,29 then precipitate at the P123/GO interface, and finally transforms into a 2D silica film with fingerprint-like parallel mesopores (Fig. 5c and d).
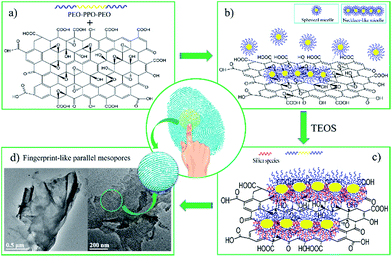 | ||
| Fig. 5 Schematic model of the co-operative assembly mechanism for the synthesis of semi-ordered 2D mesoporous silica film. | ||
As an example of application, SiO2/GO-4.23 is used as the template to synthesize 2D nitrogen-doped mesoporous carbon films (2DNMC), which are of great interests in electrochemical energy technologies.30–32
Fig. 6a reveals that the synthesized carbon is in the morphology of ultrathin film, which follows the negative replica of the silica template. This unique 2D feature is expected to yield some effect on the electrochemical behaviour in terms of the accessibility of the active species. Fig. 6b presents the polarization curve of the oxygen reduction reaction (ORR). It is seen that 2DNMC exhibits a higher onset potential than does the Pt catalyst, indicating its superior intrinsic electrocatalytic activity. Also, it is noted that the diffusion-limit current is much higher than the 3D counterpart,33–36 which may be attributed to the better accessibility of the active sites in 2DNMC. To further confirm this point, 2DNMC is used as the support for Pt. The cyclic voltammogram (CV) of the Pt/2DNMC for the methanol oxidation reaction (MOR) is seen in Fig. 6c. 2DNMC is found to yield a much higher peak current than the carbon black (Vulcan XC-72, Cabot Co.), confirming its superior ability for the methanol transfer. In addition, 2DNMC endows Pt with a better kinetics and anti-poisoning ability for the MOR, which should be attributed to the enriched electrochemically active functional groups on its surface.33–36 The above findings consolidate that the ultrathin 2D mesoporous film enables both full utilization of the catalytically active site and facile mass transfer of the active species, which are highly desirable in practical applications.
Conclusions
A novel ultrathin 2D silica film with fingerprint-like parallel mesopores was synthesized by using the dual templates of GO and P123, of which the mesopores originated from the template P123 and the 2D morphology from the template GO. The type and concentration of the template were extensively explored to clarify the formation mechanism. And it was found that the morphology closely depended on both the type of the functional groups and the concentration of the template graphene. Accordingly, it is proposed that the formation proceeds by the so-called cooperative assembly mechanism, in which the competition among the three components (TEOS, GO and P123) determines the assembly and the final morphology/structure. As an application, the silica film was used as the template to synthesize 2D nitrogen-doped mesoporous carbon film. The 2DNMC film was featured by an ultrathin thickness (1.1 nm), high specific surface area and accessible mesopore surface, enabling itself a good candidate as both the catalyst and support. Electrochemical test confirmed that as either the catalyst itself or the support for Pt, the 2DNMC film yielded superior kinetic and diffusion-limit currents than did the commercial Pt/C catalyst for the oxygen reduction reaction and methanol oxidation reaction. The above findings consolidate that the unique 2D mesoporous structure enables both full utilization of the catalytically active site and facile mass transfer of the active species, which are highly desirable in the electrochemical energy conversion and storage.Acknowledgements
The work described in this paper was jointly supported by the National Natural Science Foundation of China (No. 21476087, 21576101), Innovation Project of Guangdong Department of Education (No. 2014KTSCX016), the Science & Technology Research Project of Guangdong Province (No. 2013B010405005, 2014A010105041), and the Fundamental Research Funds for the Central Universities.References
- V. Georgakilas, M. Otyepka, A. B. Bourlinos, V. Chandra, N. Kim, K. C. Kemp, P. Hobza, R. Zboril and K. S. Kim, Chem. Rev., 2012, 112, 6156 CrossRef CAS PubMed.
- A. Saito, S.-i. Yamamoto and Y. Nishina, RSC Adv., 2014, 4, 59835 RSC.
- M. Ghidiu, M. R. Lukatskaya, M. Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78 CAS.
- B. Yu, Y. Shi, B. Yuan, L. Liu, H. Yang, Q. Tai, S. Lo, L. Song and Y. Hu, RSC Adv., 2015, 5, 13502 RSC.
- X. J. Zhou, J. L. Qiao, L. Yang and J. J. Zhang, Adv. Energy Mater., 2014, 4, 1301523 CrossRef.
- J. Zhang, Y. Xu, Z. Liu, W. Yang and J. Liu, RSC Adv., 2015, 5, 54275 RSC.
- P. J. Wei, G. Q. Yu, Y. Naruta and J. G. Liu, Angew. Chem., Int. Ed., 2014, 53, 6659 CrossRef CAS PubMed.
- Y. B. Gu, J. G. Werner, R. M. Dorin, S. W. Robbins and U. Wiesner, Nanoscale, 2015, 7, 5826 RSC.
- B. Mendoza-Sánchez and Y. Gogotsi, Adv. Mater., 2016, 28, 6104 CrossRef PubMed.
- M. A. Raj and S. A. John, RSC Adv., 2015, 5, 4964 RSC.
- D. R. Dreyer, S. Park, C. W. Bielawski and R. S. Ruoff, Chem. Soc. Rev., 2010, 39, 228 RSC.
- M. M. Devi, S. R. Sahu, P. Mukherjee, P. Sen and K. Biswas, RSC Adv., 2015, 5, 62284 RSC.
- L. J. Cote, J. Kim, V. C. Tung, J. Luo, F. Kim and J. Huang, Pure Appl. Chem., 2011, 83, 95 CAS.
- S. Lee, M. Choun, Y. Ye, J. Lee, Y. Mun, E. Kang, J. Hwang, Y. H. Lee, C. H. Shin, S. H. Moon, S. K. Kim, E. Lee and J. Lee, Angew. Chem., Int. Ed., 2015, 54, 9230 CrossRef CAS PubMed.
- Y. Hou, T. Huang, Z. Wen, S. Mao, S. Cui and J. Chen, Adv. Energy Mater., 2014, 4, 1400337 CrossRef.
- T. Kavinkumar, D. Sastikumar and S. Manivannan, RSC Adv., 2015, 5, 10816 RSC.
- J. Kim, L. J. Cote and J. Huang, Acc. Chem. Res., 2012, 45, 1356 CrossRef CAS PubMed.
- S. Yang, X. Feng, L. Wang, K. Tang, J. Maier and K. Mullen, Angew. Chem., Int. Ed., 2010, 49, 4795 CrossRef CAS PubMed.
- S. K. Movahed, M. Shariatipour and M. Dabiri, RSC Adv., 2015, 5, 33423 RSC.
- D. Zhao, J. Feng, Q. Huo, N. Melosh, G. H. Fredrickson, B. F. Chmelka and G. D. Stucky, Science, 1998, 279, 548 CrossRef CAS PubMed.
- D. Y. Zhao, J. Y. Sun, Q. Z. Li and G. D. Stucky, Chem. Mater., 2000, 12, 275 CrossRef CAS.
- S. Kang, Y. B. Chae and J. S. Yu, J. Nanosci. Nanotechnol., 2009, 9, 527 CrossRef CAS PubMed.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. Sing, Pure Appl. Chem., 2015, 87, 1051 CrossRef CAS.
- J. P. Thielemann, F. Girgsdies, R. Schlogl and C. Hess, Beilstein J. Nanotechnol., 2011, 2, 110 CrossRef CAS PubMed.
- S. Ruthstein, V. Frydman, S. Kababya, M. Landau and D. Goldfarb, J. Phys. Chem. B, 2003, 107, 1739 CrossRef CAS.
- S. Ruthstein, J. Schmidt, E. Kesselman, Y. Talmon and D. Goldfarb, J. Am. Chem. Soc., 2006, 128, 3366 CrossRef CAS PubMed.
- M. R. Munch and A. P. Gast, Macromolecules, 1988, 21, 1360 CrossRef CAS.
- K. Mortensen and J. S. Pedersen, Macromolecules, 1993, 26, 805 CrossRef CAS.
- D. Y. Zhao, Q. S. Huo, J. L. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024 CrossRef CAS.
- W. Guo, X. Li, D. H. L. Ng and J. Ma, RSC Adv., 2015, 5, 96681 RSC.
- K. G. Qu, Y. Zheng, S. Dai and S. Z. Qiao, Nanoscale, 2015, 7, 12598 RSC.
- D. Li, C. Yu, M. Wang, Y. Zhang and C. Pan, RSC Adv., 2014, 4, 55394 RSC.
- K. Wan, Z. P. Yu and Z. X. Liang, Catalysts, 2015, 5, 1034 CrossRef CAS.
- K. Wan, Z. P. Yu, X. H. Li, M. Y. Liu, G. Yang, J. H. Piao and Z. X. Liang, ACS Catal., 2015, 5, 4325 CrossRef CAS.
- K. Wan, G. F. Long, M. Y. Liu, L. Du, Z. X. Liang and P. Tsiakaras, Appl. Catal., B, 2015, 165, 566 CrossRef CAS.
- G. F. Long, K. Wan, M. Y. Liu, X. H. Li, Z. X. Liang and J. H. Piao, Chin. J. Catal., 2015, 36, 1197 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and additional data. See DOI: 10.1039/c6ra16272j |
| This journal is © The Royal Society of Chemistry 2016 |