A Pb2+-binding polychelatogen derived from thionated lactide†
A. Mangalum ,
F. Boadi,
S. A. Masand,
R. A. Lalancette and
A. Pietrangelo*
,
F. Boadi,
S. A. Masand,
R. A. Lalancette and
A. Pietrangelo*
Department of Chemistry, Rutgers University-Newark, 73 Warren Street, Newark, New Jersey 07102, USA. E-mail: a.pietrangelo@rutgers.edu
First published on 1st August 2016
Abstract
The synthesis and characterization of a polychelatogen derived from thionated lactide is reported. The four step synthesis from L-LA requires thionation and thia-Diels–Alder steps to afford a highly strained spiro-lactone adduct that is amenable to ring-opening metathesis polymerization. Saponification of the polymer affords a polyanion that exhibits a high affinity for toxic Pb2+ in aqueous solutions, results that we attribute to thioether and hydroxy carboxylate chelating moieties that are integrated into the polymer backbone and residues respectively.
Polychelatogens are a class of non-crosslinked water-soluble polymers that exhibit metal ion complexation capabilities.1 Materials such as these belong to a broader set of polymer-based reagents2 that are designed to sequester heavy metal contaminants in water resources that pose a risk to human health.3 In light of ongoing efforts to improve water quality4 in parts of the world where potable water is scarce,5 the quest for improved metal-sequestration technologies remains increasingly important if inexpensive and clean water is to be available on a global scale. In this communication, we report on a novel polychelatogen comprised entirely of thioether6 and hydroxy carboxylate7 chelators that are well known for their affinity to coordinate metal ions. Key to this work is the synthesis of a mono-thionated lactide that is capable of thia-Diels–Alder cycloadduct formation with cyclopentadiene. Ring-opening metathesis polymerization (ROMP) of the norbornene-like monomer affords a polythioether with spiro-lactone residues that are saponified to their corresponding hydroxy carboxylates. The polyanion is shown to possess a high affinity for Pb2+ in aqueous solution, results that we attribute to a coordination site architecture that employs both the polymer backbone and anionic residue of each constituent repeat unit.
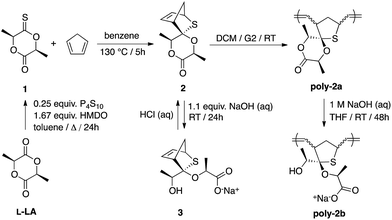
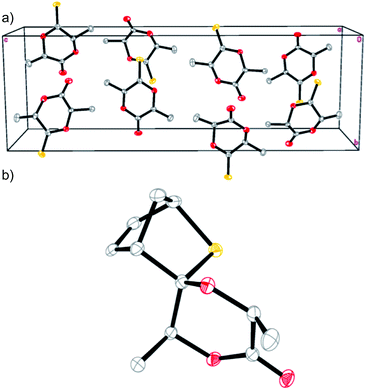
While the ring-opening polymerization of lactide (LA) to poly(lactide) (PLA) is well documented,8 the derivatization of lactide as a platform to other polymers is extremely rare.9 In this work, thionoester 1 was prepared according to Scheme 1 by reacting L-LA with a reagent combination of phosphorous pentasulfide (P4S10) and hexamethyldisiloxane (HMDO).10 Single-crystal X-ray diffraction (XRD) studies confirmed the proposed structure, revealing two independent molecules in the asymmetric unit (Z = 8, Fig. 1a) with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S (ca. 1.617(2) and 1.621(2) Å) and C
S (ca. 1.617(2) and 1.621(2) Å) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond distances (ca. 1.193(3) and 1.196(3) Å) that are in line with literature values.11 Likewise, the distinct methine and methyl environments of 1 are supported by the: (i) quartet (δ = ca. 4.94–5.06 ppm) and doublet (δ = ca. 1.56–1.77 ppm) pairs observed in the 1H NMR spectrum (Fig. S1†) and (ii) 13C resonances at δ = ca. 211.5 and 167.6 ppm (Fig. S2†) that are indicative of thionoester and ester carbonyl groups respectively. Perhaps most importantly, characterization by way of chiral high-performance liquid chromatography (Fig. S7 and S8†) and polarimetry indicate that epimerization does not occur to a significant extent during the process of thionation, hence enantiomerically pure and racemic 1 can be prepared directly from the appropriate lactide stereoisomer(s).12
O bond distances (ca. 1.193(3) and 1.196(3) Å) that are in line with literature values.11 Likewise, the distinct methine and methyl environments of 1 are supported by the: (i) quartet (δ = ca. 4.94–5.06 ppm) and doublet (δ = ca. 1.56–1.77 ppm) pairs observed in the 1H NMR spectrum (Fig. S1†) and (ii) 13C resonances at δ = ca. 211.5 and 167.6 ppm (Fig. S2†) that are indicative of thionoester and ester carbonyl groups respectively. Perhaps most importantly, characterization by way of chiral high-performance liquid chromatography (Fig. S7 and S8†) and polarimetry indicate that epimerization does not occur to a significant extent during the process of thionation, hence enantiomerically pure and racemic 1 can be prepared directly from the appropriate lactide stereoisomer(s).12
In a seminal work reported by Jing and Hillmyer,9a lactide-derived (6S)-3-methylene-6-methyl-1,4-dioxane-2,5-dione was heated with cyclopentadiene to afford a diastereotopic mixture of bicyclic Diels–Alder products that were used to prepare PLA composites with improved toughness. In this work, heating 1 with cyclopentadiene13 affords a mixture of thia-Diels–Alder products from which the major cycloadduct 2 can be separated via crystallization from hot hexanes. X-ray crystallographic data (Fig. 1b) revealed that the sulfur atom is located over the least sterically encumbered face of the lactone ring, indicating a mechanism whereby the methyl groups of the dieneophile are positioned on the same side as the approaching diene. Despite the steric congestion about the lactone moiety, its conversion into the hydroxy carboxylate derivative was demonstrated as a proof-of-principle by saponifying 2 in aqueous NaOH (1.1 mol equiv. NaOH) until its complete dissolution. The identity of 3 was unequivocally confirmed by NMR spectroscopy (Fig. S11–S14†) and high-resolution electrospray ionization mass spectrometry where peak masses of ca. 289.049, 555.111, and 821.172 are consistent with monomer (calcd for [3 + Na]+, 289.048), dimer (calcd for [(3)2 + Na]+, 555.107), and trimer adducts (calcd for [(3)3 + Na]+, 821.165) with an additional sodium ion (Fig. S15†). Incredibly, treatment of 3 with 1 M aqueous HCl regenerated 2 quantitatively (Fig. S16†) suggesting that the coordination sites in our target polymer may be deactivated upon application of a pH stimulus.
Encouraged by a report on the synthesis of sodium poly(7-oxanorbornene-2-carboxylate) from its methyl ester precursor,14 we elected to prepare polyanion poly-2b by saponification of poly-2a (Scheme 1). In this regard, monomer 2 was polymerized by ROMP using 2nd generation Grubbs' catalyst (Ru)15 at monomer-to-catalyst ratios (i.e. [2]0/[Ru]0) listed in Table 1. In line with the proposed structure, the 1H NMR spectra of poly-2a (Fig. S17–S20†) possess broad resonances at δ ca. 5.73 ppm that are consistent with the methylylidene proton environments common among norbornene-based polymers.16 It should be noted that while GPC traces (Fig. S21–S24†) are predominantly monomodal with dispersities ĐM in the range of 2.0–2.2, molecular weight control by way of adjusting [2]0/[Ru]0 was not observed under the conditions employed here. Nonetheless, the thermal properties among the high molecular weight polymers are near identical with glass transition temperatures Tgs at ca. 142 °C and thermal decomposition temperatures Tdecs spanning ca. 306–310 °C (Table 1). Poly-2a was subsequently saponified into the polyanion poly-2b by stirring in a THF/aqueous NaOH (1 M) mixture followed by dialysis against a 3.5 kDa MWCO in deionized water to remove excess hydroxide anion.
| [2]0/[Ru]0 | Mna (kg mol−1) | ĐM | Tgb (°C) | Tdecc (°C) |
|---|---|---|---|---|
| a Determined by GPC (relative to polystyrene in THF).b Glass transition temperature, second heating curve, determined by DSC.c Decomposition temperature, onset, determined by TGA. | ||||
| 100 | 51.1 | 2.1 | 142 | 306 |
| 200 | 68.6 | 2.1 | 142 | 306 |
| 400 | 135.4 | 2.0 | 141 | 308 |
| 800 | 142.8 | 2.2 | 141 | 310 |
In our preliminary investigation into the liquid-phase polymer-based retention (LPR)1a of Pb2+, aqueous solutions of poly-2b (2 mL, [poly-2b] = ca. 1 mg mL−1) and Pb2+ (1 mL, [Pb2+] = ca. 30 ppm) were combined ([poly-2b], ca. 0.67 mg mL−1; [Pb2+]0, ca. 10 ppm) and stirred under ambient conditions for 90 min followed by centrifugation through a commercially available Amicon Ultra filter equipped with a regenerated cellulose membrane (3 kDa MWCO). Filtrate analysis by atomic absorption spectroscopy revealed no detectable Pb2+ in these solutions (Fig. S36,† n = 3), even after several retentate washes (e.g., 5 × 3 mL) with deionized water (Fig. S37†). Indeed, filtrates from control experiments employing polymer-free solutions were found to possess Pb2+ concentrations of ca. 9.57 ± 0.08 ppm (Fig. S38,† n = 3) indicating that the cellulose membrane did not play a significant role in Pb2+ binding. As anticipated from earlier results, washing the membrane with three aliquots of 1 M HCl (3 mL) afforded filtrates with [Pb2+] of 7.09, 1.63, and 0.21 ppm respectively indicating that Pb2+ can be released from the polymer upon treatment with aqueous acid. As an extension of this work, filtrates from aqueous formulations ([poly-2b] = ca. 0.1 mg mL−1), [Pb2+]0 (ca. 9 ppm) spiked with: (i) Na+ (ca. 10 ppm), (ii) K+ (ca. 10 ppm) or (iii) Ca2+ (ca. 10 ppm) were found to have Pb2+ concentrations of 0.12 ± 0.01, 0.14 ± 0.01, and 0.21 ± 0.06 ppm respectively (Fig. S39,† n = 3), indicating that these alkali/alkaline earth metal ions commonly found in natural waters do not impede Pb2+ coordination to a significant extent at these concentrations.
To gain further insight into Pb2+ uptake by poly-2b, Pb2+ retention (%) was measured as a function of the initial Pb2+ concentration [Pb2+]0 (Fig. 2). Indeed, a near quantitative retention of Pb2+ by the polychelatogen ([poly-2b] = ca. 0.1 mg mL−1). pH = ca. 6, n = 3 in solutions up to [Pb2+] ca. 50 ppm was observed, after which the Pb2+ retention drops considerably as the retentate solutions become turbid. Be that as it may, the binding capacity Pb2+ in milligrams per gram of poly-2b at [Pb2+]0 = 150 ppm is ca. 2481.9 ± 158.4 mg (Pb2+) per g (poly-2b), a value that is among the highest reported to date.2b,17 Moreover, [poly-2b] concentrations could be increased to promote the near quantitative retention of Pb2+ from solutions of ca. [Pb2+]0 = 100 ppm (Fig. S40†), indicating that the LPR system can be optimized to enhance performance.
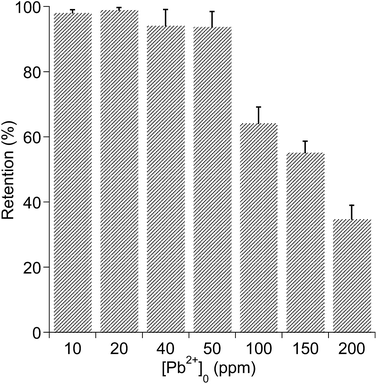 | ||
| Fig. 2 Retention (%) of Pb2+ as a function of [Pb2+]0. Data is reported as a mean with standard deviation (n = 3). [poly-2b] = 0.033 mg mL−1, pH = ca. 6. | ||
In summary, we report on the synthesis and characterization of a thionated lactide and its conversion to a spiro-lactone adduct that can be isolated in an isomerically pure form. The norbornene-like monomer can be polymerized and subsequently saponified into a poly(thioether) with hydroxy carboxylate residues, moieties that contribute to the polymer's Pb2+-binding capacity that is to the best of our knowledge, among the highest reported to date. On the bases of the results reported here, binding studies as a function of mixed (heavy) metal ion composition, pH, polymer stereochemistry, and comonomer composition are currently underway.
Acknowledgements
The authors thank Prof. Stacey Brenner-Moyer and her group for the use of their LC instrument. The authors also thank Rutgers University for financial support and the NSF for funds used to purchase our Bruker ASCEND 500 MHz spectrometer (NSF MRI 1229030).References
- (a) B. Y. Spivakov, K. Geckeler and E. Bayer, Nature, 1985, 315, 313–315 CrossRef CAS; (b) K. E. Geckeler, V. M. Shkinev and B. Y. Spivakov, Angew. Makromol. Chem., 1987, 155, 151–161 CrossRef CAS; (c) M. Tülü and M. Senel, J. Appl. Polym. Sci., 2008, 109, 2808–2814 CrossRef.
- For examples, see: (a) S. D. Alexandratos and X. Zhu, New J. Chem., 2015, 39, 5366–5373 RSC; (b) S. D. Alexandratos and S. Natesan, Macromolecules, 2001, 34, 206–210 CrossRef CAS; (c) C. A. Bell, S. V. Smith, M. R. Whittaker, A. K. Whittaker, L. R. Gahan and M. J. Monteiro, Adv. Mater., 2006, 18, 582–586 CrossRef CAS; (d) B. L. Rivas and A. Maureira, Inorg. Chem. Commun., 2007, 10, 151–154 CrossRef CAS; (e) E. Ramírez, S. G. Burillo, C. Barrera-Díaz, G. Roa and B. Bilyeu, J. Hazard. Mater., 2011, 192, 432–439 CrossRef PubMed; (f) T. Tomida, K. Hamaguchi, S. Tunashima, M. Katoh and S. Masuda, Ind. Eng. Chem. Res., 2001, 40, 3557–3562 CrossRef CAS.
- L. Järup, Br. Med. Bull., 2003, 68, 167–182 CrossRef.
- World Health Organization, Guidelines for Drinking-water Quality, Gutenberg, Malta, 4th edn, 2011 Search PubMed.
- (a) M. Elimelech and W. A. Phillip, Science, 2011, 712–717 CrossRef CAS PubMed; (b) M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, J. Mariñas and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef CAS PubMed; (c) M. R. Hartono, A. Assaf, G. Thouand, A. Kushmaro, X. Chen and R. S. Marks, Water, Air, Soil Pollut., 2015, 226, 1–11 CrossRef CAS.
- (a) S. G. Murray and F. R. Hartley, Chem. Rev., 1981, 81, 365–414 CrossRef CAS; (b) A. M. Masdeu-Bultó, M. Diéguez, E. Martin and M. Gómez, Coord. Chem. Rev., 2003, 242, 159–201 CrossRef.
- (a) S. Paria, S. Chatterjee and T. K. Paine, Inorg. Chem., 2014, 53, 2810–2821 CrossRef CAS PubMed; (b) T. K. Paine, S. Paria and L. Que Jr, Chem. Commun., 2010, 46, 1830–1832 RSC; (c) C. M. Cawich, A. Ibrahim, K. L. Link, A. Bumgartner, M. D. Patro and S. N. Mahapatro, Inorg. Chem., 2003, 42, 6458–6468 CrossRef CAS PubMed; (d) R. Codd and P. A. Lay, J. Am. Chem. Soc., 1999, 121, 7864–7876 CrossRef CAS; (e) S. M. Saadeh, M. S. Lah and V. L. Pecoraro, Inorg. Chem., 1991, 30, 8–15 CrossRef CAS; (f) E. B. Kipp and R. A. Haines, Inorg. Chem., 1972, 11, 271–276 CrossRef CAS; (g) R. A. Haines and A. A. Smith, Inorg. Chem., 1973, 12, 1426–1428 CrossRef CAS; (h) F. Cariati, F. Morazzoni and G. M. Zanderighi, Inorg. Chim. Acta, 1977, 21, 133–1440 CrossRef CAS; (i) A. Fishinger, A. Sarapu and A. Companion, Can. J. Chem., 1969, 47, 2629–2637 CrossRef; (j) Y. Zhang, I. Karatchevtseva, F. Kadi, B. Yoon, J. R. Price, F. Li and G. R. Lumpkin, Polyhedron, 2015, 87, 377–382 CrossRef CAS.
- (a) Y. Sarazin and J. F. Carpentier, Chem. Rev., 2015, 115, 3564–3614 CrossRef CAS PubMed; (b) J. M. Becker, R. J. Poundera and A. P. Dove, Macromol. Rapid Commun., 2010, 31, 1923–1937 CrossRef CAS PubMed; (c) O. Dechy-Cabaret, B. Martin-Vaca and D. Bourissou, Chem. Rev., 2004, 104, 6147–6176 CrossRef CAS PubMed; (d) M. J. Stanford and A. P. Dove, Chem. Soc. Rev., 2010, 39, 486–494 RSC; (e) C. M. Thomas, Chem. Soc. Rev., 2010, 39, 165–173 RSC.
- (a) F. Jing and M. A. Hillmyer, J. Am. Chem. Soc., 2008, 130, 13826–13827 CrossRef CAS PubMed; (b) G. Fiore, F. Jing, V. G. Young Jr, C. J. Cramer and M. A. Hillmyer, Polym. Chem., 2010, 1, 870–877 RSC; (c) J. A. Castillo, D. E. Borchmann, A. Y. Cheng, Y. Wang, C. Hu, A. García and M. Weck, Macromolecules, 2012, 45, 62–69 CrossRef CAS PubMed; (d) G. M. Miyake, Y. Zhang and E. Y.-X. Chen, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 1523–1532 CrossRef CAS; (e) T. C. Mauldin, J. T. Wertz and D. J. Boday, ACS Macro Lett., 2016, 5, 544–546 CrossRef CAS.
- (a) T. J. Curphey, J. Org. Chem., 2002, 67, 6461–6473 CrossRef CAS PubMed; (b) T. J. Curphey, Tetrahedron Lett., 2002, 43, 371–373 CrossRef CAS.
- F. H. Allen, O. Kennard and D. G. Watson, J. Chem. Soc., Perkin Trans. 2, 1987, S1–S19 RSC.
- Trace amounts of dithionated lactide can be isolated during the purification of 1. See ESI† for details.
- V. M. Timoshenko, S. A. Siry, A. B. Rozhenko and Y. G. Shermolovich, J. Fluorine Chem., 2010, 131, 172–183 CrossRef CAS.
- (a) M. Wathier, B. A. Lakin, P. N. Bansal, S. S. Stoddart, B. D. Snyder and M. W. Grinstaff, J. Am. Chem. Soc., 2013, 135, 4930–4933 CrossRef CAS PubMed; (b) M. Wathier, S. S. Stoddart, M. J. Sheehy and M. W. Grinstaff, J. Am. Chem. Soc., 2010, 132, 15887–15889 CrossRef CAS PubMed.
- M. Scholl, S. Ding, C. W. Lee and R. H. Grubbs, Org. Lett., 1999, 1, 953–965 CrossRef CAS PubMed.
- For examples, see: (a) H. A. Kang, H. E. Bronstein and T. M. Swager, Macromolecules, 2008, 41, 5540–5547 CrossRef CAS; (b) H. Gu, R. Ciganda, R. Hernandez, P. Castel, P. Zhao, J. Ruiz and D. Astruc, Macromolecules, 2015, 48, 6071–6076 CrossRef CAS; (c) D. J. Liaw, J. S. Tsai and P. L. Wu, Macromolecules, 2000, 33, 6925–6929 CrossRef CAS.
- For examples, see: (a) J. J. Alcaraz-Espinoza, A. E. Chávez-Guajardo, J. C. Medina-Llamas, C. A. S. Andrade and C. P. de Melo, ACS Appl. Mater. Interfaces, 2015, 7, 7231–7240 CrossRef CAS PubMed; (b) C. J. Madadrang, H. Y. Kim, G. Gao, N. Wang, H. Feng, M. Gorring, M. L. Kasner and S. Hou, ACS Appl. Mater. Interfaces, 2012, 4, 1186–1193 CrossRef CAS PubMed; (c) Y. L. F. Musico, C. M. Santos, M. L. P. Dalida and D. F. Rodrigues, J. Mater. Chem. A, 2013, 1, 3789–3796 RSC; (d) E. Ramirez, S. G. Burillo, C. B. Diaz, G. Roa and B. Bilyeu, J. Hazard. Mater., 2011, 192, 432–439 CrossRef CAS PubMed; (e) X. Wang, M. Min, Z. Liu, Y. Yang, Z. Zhou, M. Zhu, Y. Chen and B. S. Hsiao, J. Membr. Sci., 2011, 379, 191–199 CrossRef CAS; (f) A. Mahapatra, B. G. Mishra and G. Hota, J. Hazard. Mater., 2013, 258–259, 116–123 CrossRef CAS PubMed; (g) H. Bessbousse, T. Rhlalou, J. F. Verchere and L. Leburn, J. Membr. Sci., 2008, 307, 249–259 CrossRef CAS; (h) Y.-H. Li, J. Ding, Z. Luan, Z. Di, Y. Zhu, C. Xu, D. Wu and B. Wei, Carbon, 2003, 41, 2787–2792 CrossRef CAS; (i) S. Tolani, A. Mugweru, M. Craig and A. K. Wanekaya, J. Appl. Polym. Sci., 2010, 116, 308–313 CrossRef CAS; (j) E. D. Pereira, D. Homper, J. Sanchez and B. Rivas, J. Chil. Chem. Soc., 2015, 60, 3054–3058 CrossRef CAS; (k) R. Yang, K. B. Aubrecht, H. Ma, R. Wang, R. B. Grubbs, B. S. Hsiao and B. Chu, Polymer, 2014, 55, 1167–1176 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic details, characterization, and X-ray crystallographic data. CCDC 1477173–1477175. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra16230d |
| This journal is © The Royal Society of Chemistry 2016 |