Toxicity reduction of imidazolium-based ionic liquids by the oxygenation of the alkyl substituent†
M. Vraneš*a,
A. Tota,
S. Jovanović-Šantaa,
M. Karamanb,
S. Dožića,
K. Tešanovićb,
V. Kojićc and
S. Gadžurića
aUniversity of Novi Sad, Faculty of Sciences, Department of Chemistry, Biochemistry and Environmental Protection, Trg D. Obradovića 3, 21000 Novi Sad, Serbia. E-mail: milan.vranes@dh.uns.ac.rs; Fax: +381 21 454 065; Tel: +381 21 4852744
bUniversity of Novi Sad, Faculty of Sciences, Department of Biology and Ecology, Trg Dositeja Obradovića 3, 21000 Novi Sad, Serbia
cInstitute of Oncology Sremska Kamenica, Institutski Put 4, 21204 Sremska Kamenica, Serbia
First published on 28th September 2016
Abstract
In this work, five different salicylate based ionic liquids were prepared in order to study their toxicity: 1-butyl-3-methylimidazolium salicylate, [bmim][Sal], 1-(4-hydroxy-2-oxybutyl)-3-methylimidazolium salicylate, [OHC2OC2mim][Sal], 1-(3-hydroxypropyl)-3-methylimidazolium salicylate, [OHC3mim][Sal], 1-ethoxyethyl-3-methylimidazolium salicylate, [C2OC2mim][Sal] and imidazolium salicylate [Im][Sal]; aquatic organisms (Artemia salina) and a human non-tumor cell line (normal fetal lung fibroblasts, MRC-5) were also used in the investigation. The introduction of polar groups (in the form of hydroxide and/or ether group) into the alkyl side chain of the imidazolium cation, and their influence on the reduction of the ionic liquid's toxicity, were also demonstrated. The results indicate that the toxicity against A. salina and cytotoxicity against the healthy cell line of lipophobic ionic liquids were significantly lower than for the non-functionalized analogues, and are the same order of magnitude as the reference standard sodium salicylate. These facts open up the possibility of designing new non-toxic ionic liquids that can be used as active pharmaceutical ingredients in liquid form, adjusting only the lipophilicity of the cations by introducing polar oxygen groups into the side alkyl chain of the cation.
1. Introduction
Pharmaceutical companies currently rely on crystalline solid forms for the delivery of active pharmaceutical ingredients (APIs) because of their high purity, thermal stability, synthetic methods and easy handling.1–3 Nowadays, many therapeutics, as well as pharmaceutical formulations in the preclinical phase of development, are used in the form of salts, due to their better physical properties, better solubility, bioavailability, permeability and drug delivery potential.2–6 Pharmacokinetic features of the APIs in the form of the salts directly depend on their absorption mechanism and lipophilicity. The main problem when salts are applied as APIs is their polymorphism or pseudopolymorphism, since each form shows different solubility and bioavailability.1,2,5,6 To overcome these problems, the use of the liquid forms of the drugs has been proposed. Thus, ionic liquids (ILs), salts with melting temperatures below 100 °C, with biologically active components (cation and/or anion), were recently studied as potential APIs.6–13The ionic liquids are known as salts with tunable properties and high thermal stability, which give new perspective in the pharmaceutical industry. There are several strategies as to how ionic liquids can be used as APIs:8,13–18
(1) API-ILs as pharmaceuticals prepared as single or dual-active liquid salts of APIs, by liquefaction using oligomeric ions or by the formation of liquid co-crystals.
(2) Synthesis of new API-ILs starting from existing API-ILs.
(3) ILs for solubilization of poorly soluble drugs, including (i) APIs solubilized within micelles acting as reservoirs for controlled release, (ii) IL-assisted, non-aqueous microemulsions stabilized by the addition of external surfactant, or (iii) ILs designed as hydrophilic–lipophilic balanced (HLB) hydrotropes that keep the APIs dissolved once added to water.
Application of the ionic liquids as potential drugs is still restricted, due to the lack of data concerning their toxicity and biodegradability.8,19,20 It has been shown that many commercial ILs are toxic. Thus, the synthesis of the new ILs with environmentally friendly cations and biologically active anions, such as aminoacids is proposed. For this purpose, many choline-based ILs were synthesized, but most of them show thermal, hydrolytic and electrochemical instability.21–24 On the other hand, commercially available imidazolium ILs are more stable, but more toxic, which greatly reduce their applications. One of the strategies in preparing low-toxic ILs is reducing the toxicity of the imidazolium ion by fine tuning the essential properties by the variation of the alkyl substituents at position N1 or N3 of the imidazolium ring.25 Introducing oxygen atoms into the side alkyl chain decreases lipophilicity, due to the presence of the polar groups, while simultaneously decreasing the toxicity of the ILs.25–27 Also, the presence of the ether and/or hydroxide functions increases the solubility of the ILs in water and the solubility of the salts in ILs, promoting them as excellent candidates for API-ILs synthesis.26,28
The second strategy is to obtain biologically active ILs by selection of the adequate anion. One of the best studied anions is salicylate, which, in the form of acetylsalicylic acid (aspirin), is one of the most commonly used medicaments in the world.5,7,29,30 It is well known that the aspirin is only partially soluble in water (0.33 g in 100 mL of water) and in the acidic stomach environment, which results in undissolved particles adhering to the gastrointestinal mucosa causing irritation and gastric distress.29–31 It is obvious that better drug delivery can be achieved by applying the salicylate in the liquid form. Salicylic acid is also known as a cytotoxin with a prominent anticancer effect. Also, some salicylate based ionic liquids show luminescence properties,32 which facilitate their tracking and marking in the body.
Taking all the above mentioned facts into account, we synthesized a series of ionic liquids with imidazolium cations functionalized with ether and/or hydroxide groups and salicylate anions, in order to study their toxicity and compare the obtained results with the unsubstituted analogues of 1-butyl-3-methylimidazolium salicylate, as reported herein. In this way, the influence of the oxygen functions in the substituent on the imidazole ring on the ILs toxicity can be discussed.
2. Experimental section
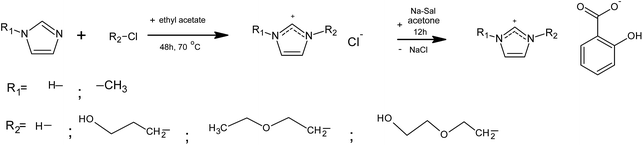
All chemicals for ILs synthesis were used as purchased from the manufacturer, without further purification: 1-methylimidazole (Sigma Aldrich, CAS number: 616-47-7, ω ≥ 0.99), 2-chloroethyl ether (Sigma Aldrich, CAS number: 628-34-2, ω ≥ 0.99), 3-chloro-1-propanol (Sigma Aldrich, CAS number: 627-30-5, ω ≥ 0.98), 2-(2-chloroethoxy)ethanol (Sigma Aldrich, CAS number: 628-89-7, ω ≥ 0.99), ethyl acetate (Sigma Aldrich, CAS number: 141-78-6, ω ≥ 0.998), sodium salicylate (Reanal, CAS number: 54-21-7; ω ≥ 0.995), imidazole (Sigma Aldrich, CAS number: 288-32-4, ω ≥ 0.99), N-methylimidazole (Sigma Aldrich, CAS number: 616-47-7, ω ≥ 0.99), hydrochloric acid (Sigma Aldrich, CAS number: 7647-01-0), acetone (Lachner, CAS number: 67-64-1).Five different salicylate based ionic liquids were synthesized. The synthesis of 1-butyl-3-methylimidazolium salicylate ionic liquid, [bmim][Sal], is described in our previous paper.33 Other ILs, namely: [OHC2OC2mim][Sal], [OHC3mim][Sal], [C2OC2mim][Sal] and [Im][Sal] were prepared, starting from the corresponding chloride salts: [OHC2OC2mim][Cl], [C3OHmim][Cl], [C2OC2mim][Cl] and [Im][Cl], according to the synthetic path presented in Fig. 1.
3-Chloro-1-propanol (or 2-(2-chloroethoxy)ethanol or 2-chloroethyl ether) and 1-methylimidazole were added to a round-bottom flask. Ethyl acetate was used as a solvent, and 3-chloro-1-propanol (or 2-(2-chloroethoxy)ethanol or 2-chloroethyl ether) was added in 10% excess. The mixture was kept under reflux for 48 h at 70 °C with stirring, until two phases were formed. The top phase, containing unreacted starting material was removed. The bottom phase was washed four times with new portions of ethyl acetate. The products were obtained in the liquid state, and stored under vacuum with P2O5 for the next 72 h.
Non-substituted imidazolium chloride, [Im][Cl], was synthesized by potentiometric acid–base titration. The reaction was conducted by the slow addition of HCl (0.1053 mol dm−3) to an aqueous solution of imidazole, with constant stirring until an adequate pH was achieved. Additionally, the IL was dried under vacuum for the next 24 h in order to remove water, and the obtained solid [Im][Cl] was stored under P2O5.
The obtained chloride ionic liquids were transformed into salicylates by the addition of equimolar amounts of sodium salicylate, using acetone as a solvent. The resulting solution was stirred and refluxed for 12 h. After that, the white precipitate (NaCl) was removed and the acetone solution of the ionic liquid was obtained. Acetone was removed by evaporation under vacuum at 70 °C for 1 h, achieving constant mass. The water content was determined by Karl-Fischer titration, and the chloride content by ion chromatography. It was found that the water content was less than 200 ppm and the chloride content was less than 8.3 ppm in all synthesized ionic liquids.
For additional characterization, the IR and NMR spectra (Fig. S1 and S2 in ESI†) of the newly synthesized ionic liquids were recorded. NMR spectra were recorded in D2O at 25 °C on a Bruker Advance III 400 MHz spectrometer. Tetramethylsilane was used as the accepted internal standard for calibrating the chemical shift for 1H and 13C. The 1H homodecoupling and the 2D COSY method were used routinely for the assigning of the obtained NMR spectra. 13C spectra were assigned by a selective decoupling technique.
2.1. [OHC2OC2mim][Sal]
1H NMR (D2O): 3.45 (m, 2H, NCH2CH2OCH2CH2OH); 3.58 (m, 2H, NCH2CH2OCH2CH2OH); 3.67 (s, 3H, CH3); 3.67 (t, 2H, NCH2CH2OCH2CH2OH); 4.13 (t, 2H, J = 4.7, NCH2CH2OCH2CH2OH); 6.77 (d, 1H, J3′,4′ = 8.2 Hz, H-3′); 6.10 (t, 1H, J3′,4′ = 7.5 Hz, H-5′); 7.17 and 7.25 (2xs, 2H, H-4 and H-5); 7.29 (m, 1H, H-4′); 7.67 (dd, 1H, J4′,6′ = 1.3 Hz, J5′,6′ = 7.8 Hz, H-6′); 8.44 (s, 1H, H-2).13C NMR (D2O): 38.63 (NCH3); 51.77 (NCH2CH2OCH2CH2OH); 63.08 (NCH2CH2OCH2CH2OH); 70.99 (NCH2CH2OCH2CH2OH); 74.51 (NCH2CH2OCH2CH2OH); 118.97 (C-3′); 120.75 (C-1′); 122.03 (C-5′); 125.17 and 126.07 (C-5 and C-4); 133.18 (C-6′); 136.66 (C-4′); 138.83 (C-2); 162.47 (C-2′); 177.80 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
2.2. [C2OC2mim][Sal]
1H NMR (D2O): 1.05 (t, 3H, J = 7.0 NCH2CH2OCH2CH3); 3.43 (q, 2H, J = 7.1, NCH2CH2OCH2CH3); 3.66 (t, 2H, NCH2CH2OCH2CH3); 3.73 (s, 3H, NCH3); 4.14 (t, 2H, NCH2CH2OCH2CH3); 6.80 (d, 1H, J3′,4′ = 8.3 Hz, H-3′); 6.84 (t, 1H, J3′,4′ = 7.6 Hz, H-5′); 7.23 and 7.27 (2xs, 2H, H-4 and H-5); 7.31 (m, 1H, H-4′); 7.72 (bd, 1H, J5′,6′ = 7.8 Hz, H-6′); 8.47 (s, 1H, H-2).13C NMR (D2O): 16.83 (NCH2CH2OCH2CH3); 38.39 (NCH3); 51.76 (NCH2CH2OCH2CH3); 69.39 (NCH2CH2OCH2CH3); 70.38 (NCH2CH2OCH2CH3); 74.51 (NCH2CH2OCH2CH3); 118.94 (C-3′); 120.88 (C-1′); 121.99 (C-5′); 125.12 and 126.19 (C-5 and C-4); 133.19 (C-6′); 136.59 (C-4′); 138.70 (C-2); 162.54 (C-2′); 177.71 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
2.3. [OHC3mim][Sal]
1H NMR (D2O): 2.00 (m, 2H, NCH2CH2CH2OH); 3.55 (t, 2H, J = 6.1, NCH2CH2CH2OH); 3.78 (s, 3H, NCH3); 4.15 (t, 2H, J = 7.2, NCH2CH2CH2OH); 6.84–6.94 (m, 2H, H-3′ and H-5′); 7.29 and 7.34 (2xs, 2H, H-4 and H-5); 7.40 (m, 1H, H-4′); 7.75 (d, 1H, J5′,6′ = 8.1 Hz, H-6′); 8.54 (s, 1H, H-2).13C NMR (D2O): 34.35 (NCH2CH2CH2OH); 38.39 (NCH3); 49.19 (NCH2CH2CH2OH); 60.64 (NCH2CH2CH2OH); 119.04 (C-3′); 120.74 (C-1′); 122.14 (C-5′); 124.99 and 126.33 (C-5 and C-4); 133.24 (C-6′); 136.77 (C-4′); 138.63 (C-2); 162.44 (C-2′); 178.08 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
2.4. [Im][Sal]
1H NMR (D2O): 6.35–6.45 (m, 2H, H-3′ and H-5′); 6.83–6.93 (m, 3H, H-4, H-5 and H-4′); 7.34 (dd, 1H, J5′,6′ = 7.8 Hz, J4′,6′ = 0.9 Hz, H-6′); 8.10 (s, 1H, H-2).13C NMR (D2O): 118.62 (C-3′); 120.34 (C-1′); 121.21 (C-5′); 121.62 (C-5 and C-4); 132.84 (C-6′); 135.52 (C-2); 136.26 (C-4′); 162.14 (C-2′); 177.81 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).
O).
Infrared spectra were recorded on neat samples from (4000–650) cm−1 on a Thermo-Nicolet Nexus 670 spectrometer fitted with a Universal ATR Sampling Accessory.
2.5. [OHC2OC2mim][Sal]
3500–3300 (H-bond OH); 3145 (sym. stretching ν HC((4)C(5)H)); 3094 (sym. stretching ν HC(2)); 2867 (sym. stretching ν CH3); 1584 (in-plane vibrations of imidazolium ring); 1484 (stretching ν CC); 1380 (stretching ν C–O); 1323 (in-plane bending mode δ O–H); 1125 and 1067 (stretching ν C–O from ether group); 807 (in-plane bending mode δ CC).2.6. [C2OC2mim][Sal]
3142 (sym. stretching ν HC((4)C(5)H)); 3077 (sym. stretching ν HC(2)); 2974 (asym. stretching ν CH3); 2871 (sym. stretching ν CH3) 1585 (in-plane vibrations of imidazolium ring); 1483 and 1284 (stretching ν CC); 1376 (stretching ν C–O); 1166 (stretching ν C–O); 1144 and 1026 (stretching ν C–O from ether group); 806 (in-plane bending mode δ CC);2.7. [OHC3mim][Sal]
3260 (stretching OH); 2954 (asym. stretching ν CH3); 2880 (sym. stretching ν CH3); 3081 (sym. stretching ν HC(2)); 3144 (sym. stretching ν HC((4)C(5)H)); 1585 (in-plane vibrations of imidazolium ring); 1483 and 1284 (stretching ν CC); 1376 (stretching ν C–O); 1166 (stretching ν C–O); 806 (in-plane bending mode δ CC); 702 (out-of-plane deformation γ CC).2.8. [Im][Sal]
3159 (sym. stretching ν HC((4)C(5)H)); 3047 (sym. stretching ν HC(2)); 2978 (asym. stretching ν HC(2)); 1602 (in-plane vibrations of imidazolium ring); 1488 (stretching ν CC) 1383 (stretching ν C–O); 1463 and 1346 (stretching ν C–N); 809 (in-plane bending mode δ CC); 667 (out-of-plane deformation γ CC).2.9. Toxicity assays
Cell lines and cell culture. Human non-tumor cell line (normal fetal lung fibroblasts MRC-5, ATCC CCL 171) was used in this study. Cells were grown in Dulbecco's modified Eagle's medium (DMEM) containing 4.5% of glucose. Media were supplemented with 10% of fetal calf serum (FCS, Sigma) and antibiotics: 105 IU mL−1 of penicillin and 100 μg mL−1 of streptomycin (ICN Galenika). The cell line was cultured in a flask (Costar, 25 cm2) at 37 °C in a 100% humidity atmosphere containing 5% of CO2. Only viable cells were used in the assays. Cell viability was determined by the trypan blue dye exclusion assay.
Antiproliferative activity. Antiproliferative activity of the tested salicylate derivatives was evaluated by tetrazolium colorimetric MTT ([3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]) assay.35 Cells were exposed to the test compounds over 72 h, in concentrations ranging from 10−8 to 10−4 mol L−1. The reference compound used in the MTT assay was sodium salicylate. Exponentially growing cells were harvested, counted by trypan blue dye exclusion test, seeded onto 96-well plates at a density of 5000 cells/well and allowed to stand overnight. Solutions of the tested compounds in medium (10 μmol per L per well) were added and therefore, the final concentrations ranged from 10−8 to 10−4 mol L−1. After the 72 h treatment period, cell viability was determined by the addition of 10 μL of sterile MTT solution (5 mg mL−1). The precipitated formazan crystals were solubilized with acidified 2-propanol (100 μL of 0.04 mol L−1 HCl in 2-propanol) and the absorbance was recorded (Multiscan MCC340, Labsystems) at 540 and 690 nm after a few minutes incubation at room temperature. Wells containing cells without tested compounds were used as controls. Wells without cells, containing only complete medium and MTT were used as the blank. Cytotoxicity (CI) was calculated according to the following formula:
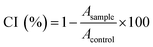 | (1) |
Data analysis. Two independent experiments were conducted in quadruplicate for each concentration of tested compound. Mean values and standard deviations (σ) were calculated for each concentration. Antiproliferative activity was expressed as IC50 value, defined as the dose of compound that inhibits cell growth by 50%. The IC50 of each tested compound was determined by median effect analysis.36
3. Results and discussion
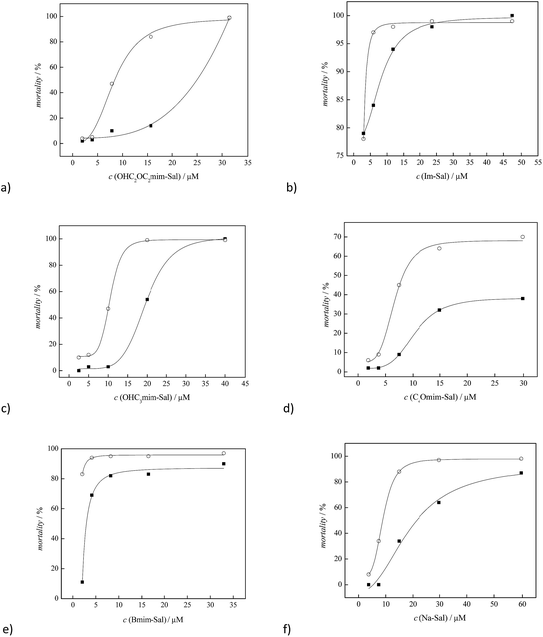
Described tests on aquatic organisms (Artemia salina) and on the human non-tumor cell line (normal fetal lung fibroblasts, MRC-5) were performed in order to investigate the influence of the alkyl chain substituent on the ILs toxicity. The assay based on toxicity against A. salina is considered rapid, convenient, low cost and one of the most reliable methods for the preliminary detection of mycotoxins, heavy metals and pesticide toxicity.34,37,38 Also, this test is often used for the general toxicity testing of compounds before their commercial use.37,39 In this work, it was used for the lethal concentration (LC50) determination.In order to investigate toxicity against healthy human cells, the MRC-5 cell line was used, which is commonly applied in vaccine development40 as a transfection host in virology research,41 and for in vitro cytotoxicity testing.42 In order to evaluate the cytotoxicity of the synthesized salicylate ionic liquids, obtained IC50 values were compared with the results obtained for non-functionalized ionic liquid [bmim][Sal] and for the sodium salicylate. The first reference compound is highly toxic, while the second one is commonly used in medicine as an analgetic, antipyretic, and non-steroidal anti-inflammatory drug that has antitumor potential, due to inducing necrosis and/or apoptosis in cancer cells.43–46
The obtained toxicity results are presented in Table 1 and in Fig. 2 and 3.
| Salt | LC50 (A. salina)/μM | IC50 (MRC-5)/μM | |
|---|---|---|---|
| a * very high toxicity at low concentrations, LC50 could not be determined.** IC50 is not detected in the investigated concentration range, meaning that these compounds can be considered as non-toxic. | |||
| 1 | Sodium salicylate | 8.87 | ** |
| 2 | [bmim][Sal] | * | 27.54 |
| 3 | [OHC2OC2mim][Sal] | 8.41 | ** |
| 4 | [C2OC2mim][Sal] | 8.18 | ** |
| 5 | [OHC3mim][Sal] | 10.18 | ** |
| 6 | [Im][Sal] | * | ** |
Ionic liquids [bmim][Sal] and [Im][Sal] showed high toxicity, even at the lowest ILs concentrations (3 μM) on A. salina larvae within the first 24 h; thus, the corresponding values of LC50 were not detected.
The introduction of oxygen in the form of a hydroxy and/or ether group into the side alkyl chain of the imidazolium ion leads to a significant reduction of the ILs toxicity to A. salina, where the LC50 values of these newly synthesized ILs after 48 h were found to be similar to the LC50 obtained for the reference sodium salicylate. A significant reduction in toxicity can be observed by comparing the results obtained for [bmim][Sal] and ILs with the oxygen in the alkyl substituent (Table 1, Fig. 2). It can be seen that introduction of the oxygen in the form of the hydroxide group in [OHC3mim][Sal], had a greater impact on reducing toxicity than the presence of oxygen in the form of the ether group in the side alkyl chain of [C2OC2mim][Sal]. This observation is in agreement with that reported by Stolte et al.27,47 In the case of [OHC2OC2mim][Sal], the introduction of the OH group in the alkyl chain with the existing ether oxygen had a lower impact on reducing the toxicity, compared to the introduction of the OH group in the non-functionalized alkyl chain.
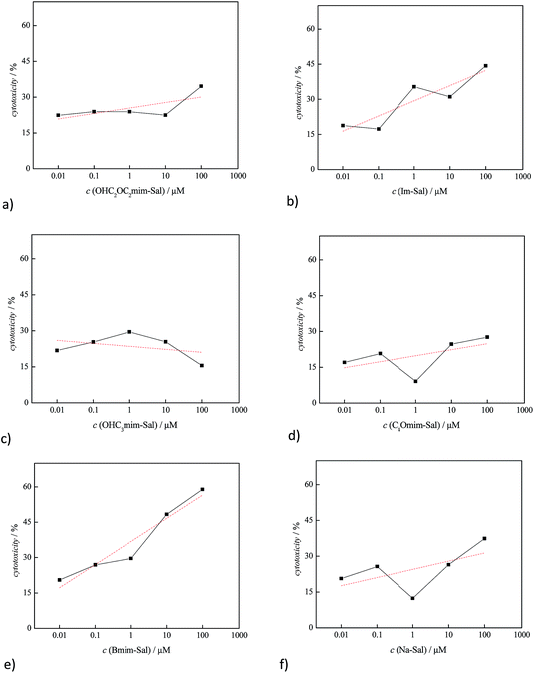
In the case of healthy lung cells, MRC-5, it was observed that only [bmim][Sal] expressed significant toxicity, its IC50 was 27.54 μM (Table 1). Otherwise, oxygen-modified ILs were not toxic against the healthy cell line MRC-5. The cytotoxicity tests were performed in the ionic liquid concentration range from 0.01 to 100 μmol mL−1 and the results are presented graphically in Fig. 3.
It is known that the structure of the cation has the greater impact on the ILs toxicity.25,27,47–49 From the results presented in this manuscript, it is obvious that the alkyl side chain is the primary factor that affects the toxicity of imidazolium based ionic liquids, i.e., a change in the polarity. This phenomenon can be explained by the assumption that the lipophilic cations are adsorbed or intercalated into the cell membrane, causing “perturbations” in the membrane (expansion or swelling, increase in fluidity, lowering of the phase transition temperature and alteration of the ion permeability of the membrane).25,49–52 The presented results indicate that the presence of a lipophilic butyl group leads to proportional destabilization of the lipid double layer membrane, which results in a linear increase of the toxicity with increasing IL concentration (Fig. 3e). Introduction of oxygen into the alkyl substituent increases the polarity of the ion, while the lipophilicity decreases. This weakens the interactions between lipid cell membrane and the ionic liquid, which significantly reduces the toxicity (Fig. 3a, c and d). The lower toxicity of the ionic liquid containing the hydroxide group in the side chain, in relation to the corresponding ether group, leads to the conclusion that the toxicity is largely affected by the type of functional group of the cation. The terminal group of the alkyl chain is the most accessible for the interactions with the cellular membrane, and its reactivity, namely hydrophilicity/hydrophobicity, is mainly responsible for the toxicity of the whole ionic liquid. The [C2OC2mim][Sal] ionic liquid has a more hydrophobic methyl group at the end of the alkyl chain, compared to [OHC3mim][Sal], where the side chain terminates with a distinctly polar OH group, thus explaining the higher toxicity of [C2OC2mim][Sal]. Also, it can be observed that additional oxygenation of the alkyl chain does not decrease the toxicity of the ionic liquids, which is in agreement with the results reported by Samori et al.53
The ionic liquids examined in this work, contain the biologically active salicylate anion and cation as drug carriers. Due to the dissociation of the ionic compounds, the cation and anion can be delivered separately into the cells. Thus, by decreasing the lipophilicity of the cation, its toxicity and bioavailability can be reduced without affecting the bioavailability of the salicylate anion.
4. Conclusions
In this paper the toxicity of newly synthesized salicylate based ionic liquids has been investigated using aquatic organisms (A. salina) and human non-tumor cell line (normal fetal lung fibroblasts, MRC-5). Also, the influence of the oxygenation (in the form of hydroxy and/or ether groups) of the alkyl side chain on the toxicity reduction was studied. The results indicate that both toxicity against A. salina and cytotoxicity against healthy cell line of lipophobic ionic liquids are significantly lower than non-functionalized analogues, and the same order of magnitude as the reference standard, sodium salicylate. The most significant impact on the reduction of the toxicity shows the introduction of a hydroxide function at the terminal position of the alkyl substituent of the imidazolium cation, while the introduction of a larger amount of oxygen does not contribute to reduced toxicity. Bearing in mind the obtained results, this opens the possibility of designing new, non-toxic ILs, adjusting only the lipophilicity of the cations by introducing polar oxygen groups into the side alkyl chain.Acknowledgements
This work was financially supported by the Ministry of Education, Science and Technological Development of Republic of Serbia under project contract ON172012.References
- S. M. Berge, L. D. Bighley and D. C. Monkhouse, J. Pharm. Sci., 1977, 66, 1 CrossRef CAS PubMed.
- A. T. M. Serajuddin, Adv. Drug Delivery Rev., 2007, 59, 603 CrossRef CAS PubMed.
- R. Banerjee, R. M. Bhatt, N. V. Ravindra and G. R. Desiraju, Cryst. Growth Des., 2005, 5, 2299 CAS.
- I. Pasquali, R. Bettini and F. Giordano, Adv. Drug Delivery Rev., 2008, 60, 399 CrossRef CAS PubMed.
- P. C. A. G. Pinto, D. M. G. P. Ribeiro, A. M. O. Azevedo, V. D. Justina, E. Cunha, K. Bica, M. Vasiloiu, S. Reisa and M. L. M. F. S. Saraiva, New J. Chem., 2013, 37, 4095 RSC.
- I. M. Marrucho, L. C. Branco and L. P. N. Rebelo, Annu. Rev. Chem. Biomol. Eng., 2014, 5, 527 CrossRef CAS PubMed.
- K. S. Egorova, M. M. Seitkalieva, A. V. Posvyatenko, V. N. Khrustalev and V. P. Ananikov, ACS Med. Chem. Lett., 2015, 6, 1099 CrossRef CAS PubMed.
- J. L. Shamshina, P. S. Barber and R. D. Rogers, Expert Opin. Drug Delivery, 2013, 10, 1367 CrossRef CAS PubMed.
- S. Bontha, A. V. Kabanov and T. K. Bronich, J. Controlled Release, 2006, 114, 163 CrossRef CAS PubMed.
- J. O. Kim, G. Sahay, A. V. Kabanov and T. K. Bronich, Biomacromolecules, 2010, 11, 919 CrossRef CAS PubMed.
- M. Kamimura, J. O. Kim, A. V. Kabanov, T. K. Bronich and Y. Nagasaki, J. Controlled Release, 2012, 160, 486 CrossRef CAS PubMed.
- O. A. Cojocaru, K. Bica, G. Gurau, A. Narita, P. D. McCrary, J. L. Shamshina, P. S. Barber and R. D. Rogers, MedChemComm, 2013, 4, 559 RSC.
- J. L. Shamshina, S. P. Kelley, G. Gurau and R. D. Rogers, Nature, 2015, 528, 188 CrossRef CAS PubMed.
- H. D. Williams, Y. Sahbaz, L. Ford, T. H. Nguyen, P. J. Scammells and C. J. Porter, Chem. Commun., 2014, 50, 1688–1690 RSC.
- M. Omar, UK J. Pharm. Biosci., 2016, 4, 41 CrossRef.
- D. Dobler, T. Schmidts, I. Klingenhofer and F. Runkel, Int. J. Pharm., 2013, 441, 620 CrossRef CAS PubMed.
- S. S. Kumar, M. Surianarayanan, R. Vijayaraghavan, A. B. Mandal and D. R. MacFarlane, Eur. J. Pharm. Sci., 2014, 51, 34–44 CrossRef CAS PubMed.
- P. D. McCrary, P. A. Beasley, G. Gurau, A. Narita, P. S. Barber, O. A. Cojocaru and R. D. Rogers, New J. Chem., 2013, 37, 2196 RSC.
- K. S. Egorova and V. P. Ananikov, ChemSusChem, 2014, 7, 336 CrossRef CAS PubMed.
- T. P. Thuy Pham, C. W. Cho and Y. S. Yun, Water Res., 2010, 44, 352 CrossRef PubMed.
- X. D. Hou, Q. P. Liu, T. J. Smith, N. Li and M. H. Zong, PLoS One, 2013, 8, 59145 Search PubMed.
- M. Petkovic, J. L. Ferguson, H. Q. Nimal Gunaratne, R. Ferreira, M. C. Leitão, K. R. Seddon, L. P. N. Rebelo and C. S. Pereira, Green Chem., 2010, 12, 643 RSC.
- P. Nockemann, B. Thijs, K. Driesen, C. R. Janssen, K. Van Hecke, L. Van Meervelt, S. Kossmann, B. Kirchner and K. Binnemans, J. Phys. Chem. B, 2007, 111, 5254 CrossRef CAS PubMed.
- K. D. Weaver, H. J. Kim, J. Sun, D. R. MacFarlane and G. D. Elliott, Green Chem., 2010, 12, 507 RSC.
- S. Stolte, M. Matzke, J. Arning, A. Böschen, W. R. Pitner, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2007, 9, 1170 RSC.
- S. Tanga, G. A. Bakerb and H. Zhao, Chem. Soc. Rev., 2012, 41, 4030 RSC.
- J. Arning, S. Stolte, A. Böschen, F. Stock, W. R. Pitner, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2008, 10, 47 RSC.
- M. Vraneš, A. Tot, S. Armaković, S. J. Armaković and S. Gadžurić, J. Chem. Thermodyn., 2016, 95, 174 CrossRef.
- K. Bica, C. Rijksen, M. Nieuwenhuyzena and R. D. Rogers, Phys. Chem. Chem. Phys., 2010, 12, 2011 RSC.
- L. Alfonso, G. Ai, R. Spitale and G. J. Bhat, Br. J. Cancer, 2014, 111, 61 CrossRef CAS PubMed.
- A. Shiotani, T. Kamada and K. Haruma, J. Gastroenterol., 2008, 43, 581 CrossRef CAS PubMed.
- P. S. Campbell, M. Yang, D. Pitz, J. Cybinska and A. V. Mudring, Chem.–Eur. J., 2014, 20, 4704 CrossRef CAS PubMed.
- M. Vraneš, S. Armaković, A. Tot, S. Papović, N. Zec, S. J. Armaković, N. Banić and B. Abramović, J. Chem. Thermodyn., 2016, 93, 164 CrossRef.
- A. L. Parra, R. S. Yhebra, I. G. Sardińas and L. I. Buela, Phytomedicine, 2001, 8, 395 CrossRef.
- T. Mossman, J. Immunol. Methods, 1983, 65, 55 CrossRef.
- A. Sotto, V. Foulongne, D. Sirot, R. Labia and J. Jourdan, Int. J. Antimicrob. Agents, 2002, 19, 75 CrossRef CAS PubMed.
- M. Hartl and H. U. Humpf, Food Chem. Toxicol., 2000, 38, 1097 CrossRef CAS PubMed.
- W. Gouveia, T. F. Jorge, S. Martins, M. Meireles, M. Carolino, C. Cruz, T. V. Almeida and M. E. M. Araújo, Chemosphere, 2014, 104, 51 CrossRef CAS PubMed.
- A. Manilal, S. Sujith, S. Kiran, J. Selvin and C. Shakir, Global J. Pharmacol., 2009, 3, 90 Search PubMed.
- A. B. Johnson and A. Lewis, Molecular Biology of the Cell, Garland Science, New York, 4th edn, 2002 Search PubMed.
- A. Stary, P. Kannouche, A. R. Lehman and A. Sarasin, J. Biol. Chem., 2003, 278, 18767 CrossRef CAS PubMed.
- D. J. S. Patinha, L. C. Tome, C. Florindo, H. R. Soares, A. S. Coroadinha and I. M. Marrucho, ACS Sustainable Chem. Eng., 2016, 4, 2670 CrossRef CAS.
- L. K. Sztriha, K. Sas and L. Vecsei, J. Neurol. Sci., 2004, 229/230, 163 CrossRef PubMed.
- N. Gong, M. Zhang, X. B. Zhang, L. Chen, G. C. Sun and T. L. Xu, Neuropharmacology, 2008, 54, 454 CrossRef CAS PubMed.
- Y. Sakaeda, M. Hiroi, T. Shimojima, M. Iguchi, H. Kanegae and Y. Ohmori, Biochem. Biophys. Res. Commun., 2006, 350, 339 CrossRef CAS PubMed.
- T. P. McDade, R. A. Perugini, F. J. Vittimberga Jr, R. C. Carrigan and M. P. Callery, J. Surg. Res., 1999, 83, 56 CrossRef CAS PubMed.
- S. Stolte, J. Arning, U. Bottin-Weber, A. Müller, W. R. Pitner, U. Welz-Biermann, B. Jastorff and J. Ranke, Green Chem., 2007, 9, 760 RSC.
- H. Konemann, Toxicology, 1981, 19, 209 CrossRef CAS PubMed.
- M. T. D. Cronin and T. W. Schultz, Sci. Total Environ., 1997, 204, 75 CrossRef CAS PubMed.
- A. P. van Wezel and A. Opperhuizen, Crit. Rev. Toxicol., 1995, 25, 255 CrossRef CAS PubMed.
- J. Ranke, S. Stolte, R. Stormann, J. Arning and B. Jastorff, Chem. Rev., 2007, 107, 2183 CrossRef CAS PubMed.
- J. Ranke, A. Müller, U. Bottin-Weber, F. Stock, S. Stolte, J. Arning, R. Stormann and B. Jastorff, Ecotoxicol. Environ. Saf., 2007, 67, 430 CrossRef CAS PubMed.
- C. Samori, D. Malferrari, P. Valbonesi, A. Montecavalli, F. Moretti, P. Galleti, G. Sartor, E. Tagliavini, E. Fabbri and A. Pasteris, Ecotoxicol. Environ. Saf., 2010, 73, 1456 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: NMR and IR spectra of newly synthesized ionic liquids. See DOI: 10.1039/c6ra16182k |
| This journal is © The Royal Society of Chemistry 2016 |