DOI:
10.1039/C6RA16023A
(Paper)
RSC Adv., 2016,
6, 84231-84235
New low polar tolane cholesterics designed for infrared applications
Received
20th June 2016
, Accepted 31st August 2016
First published on 31st August 2016
Abstract
We have designed, synthesized and evaluated the physical properties of new fluorinated phenyltolane based chiral liquid crystal materials with 2-methylbutyl terminal chain. The 2- or 2,6-fluorosubstitution in combination with the phenyltolane core brings surprising improvement of mesomorphic properties. The investigated compounds are characterized by a broad temperature range of the cholesteric (chiral nematic N*) phase, very low melting temperatures and easy thermal control of selective reflection in the near infrared region. The moderate helical twisting power β, high optical anisotropy and source of a blue phase make these compounds attractive for cholesteric mixture formulations.
Introduction
Cholesteric structures attract much attention due to their number of unique applications.1–5 Advanced studies on blue phase materials6 have also recently brought an increased interest in the chiral liquid crystal field. These fields provide fresh impact in the area of the design and synthesis of new cholesteric liquid crystals.7 Selective light reflection given by the cholesteric LCs may be placed in the UV, visible or IR wavelengths. Materials with reflection of infrared light and transparency in the visible region have already found many application areas such as an infrared-light reflective plates,8 films,9 reflectors,10 followed by numerous patent applications. They are used mainly to reduce the energy consumption or as a window heat-shielders (energy-saving smart windows).11 These and other innovative applications create a need for broadband IR reflector, where characteristic reflection bandwidth Δλ should be wide. Δλ measured as a width of the reflection bandgap at half height is normally limited to ∼100 nm in the visible spectrum. This value strongly depends on the birefringence of the material used. Therefore, continuous efforts in broadening of the reflection band bring the need for increasing the birefringence Δn of cholesteric systems.12 Increase of Δλ, obtained as a result of birefringence increase may be done only by the synthesizing tailored liquid crystal compounds. Molecules with large electron polarizability anisotropy Δα, which is induced by the presence of the long conjugated π electron bond systems, have high birefringence. The most promising are molecules with large length to breadth ratio and rigid cores composed of aromatic rings. The best ones comprise of benzene rings connected directly, or better, via the ethynyl (–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–) group.13 Highly birefringent liquid crystals are often difficult to synthesize, moreover solubility of such structures is also usually poor. For these reasons, the fabrication of novel cholesteric liquid crystals with increased birefringence, hence broad bandgap is challenging. Typical commercially available chiral compounds show rather poor solubility in the liquid crystal host (several wt%), mainly because of structural incompatibility with widely used nematic hosts. Additionally, for a vast majority of cholesteric liquid crystals used nowadays Δn is less than 0.3.14 In this work we focused on synthesis of new class of liquid crystal materials based on phenyltolane core, which assures increased birefringence Δn > 0.3. Lateral fluorine substitution, 2,6-difluorophenylacetylene unit in particular brings unique and valuable nematic stability,15–17 which in connection with a chiral centre broadens significantly the cholesteric (N*) phase range, even up to 100 K. As a chiral source (S)-2-methylbutyl alkyl chain was chosen, mainly because it's easily accessible. Although the selected chiral chain, due to lack of strong polar substituents in vicinity to asymmetric carbon, does not provide high helical twisting power (β < 20 μm−1), still great solubility and miscibility with various achiral hosts make these kind of structures promising materials. Compounds were designed to show structural similarity to widely used nematic achiral hosts, in that way the solubility and interactions with hosts could be enhanced. As a result, solutions of different concentrations of chiral compound (5 ÷ 20 wt%) were composed, which shows reflection in the near infrared region.
C–) group.13 Highly birefringent liquid crystals are often difficult to synthesize, moreover solubility of such structures is also usually poor. For these reasons, the fabrication of novel cholesteric liquid crystals with increased birefringence, hence broad bandgap is challenging. Typical commercially available chiral compounds show rather poor solubility in the liquid crystal host (several wt%), mainly because of structural incompatibility with widely used nematic hosts. Additionally, for a vast majority of cholesteric liquid crystals used nowadays Δn is less than 0.3.14 In this work we focused on synthesis of new class of liquid crystal materials based on phenyltolane core, which assures increased birefringence Δn > 0.3. Lateral fluorine substitution, 2,6-difluorophenylacetylene unit in particular brings unique and valuable nematic stability,15–17 which in connection with a chiral centre broadens significantly the cholesteric (N*) phase range, even up to 100 K. As a chiral source (S)-2-methylbutyl alkyl chain was chosen, mainly because it's easily accessible. Although the selected chiral chain, due to lack of strong polar substituents in vicinity to asymmetric carbon, does not provide high helical twisting power (β < 20 μm−1), still great solubility and miscibility with various achiral hosts make these kind of structures promising materials. Compounds were designed to show structural similarity to widely used nematic achiral hosts, in that way the solubility and interactions with hosts could be enhanced. As a result, solutions of different concentrations of chiral compound (5 ÷ 20 wt%) were composed, which shows reflection in the near infrared region.
Experimental
Synthesis
Synthetic approach described deeply in ref. 17 is generally based on the method of a copper (Li2CuCl4) catalysed cross coupling of an aryl Grignard reagents with alkyl bromides. This method is a simple modification of known procedure,18 gives much greater yields and besides of non-branched alkyl chains can be easily used for chiral branched (S-2-methylbutyl) alkyl chains incorporation as well. For the synthesis commercially available (S)-(+)-1-bromo-2-methylbutane (99% ee) was used. Following this one-pot procedure we obtained both alkyl fluorosubstituted benzene (1, 2) and 4-alkylbiphenyl (5) derivatives that were main starting materials to final mesogens' synthesis, see Fig. 1. Subsequent synthetic process was generally based on Sonogashira cross coupling reaction, after which purification of final compounds could be carried out.
 |
| | Fig. 1 Synthetic route to investigated compounds. Synthetic conditions: (a) Mg, THF anhydrous; (b) (S)-(+)-1-bromo-2-methylbutane, Li2CuCl4; (c) n-BuLi or sec-BuLi, THF anhydrous, −70 °C, then iodine; (d) I2, HIO3, CH3COOH, H2SO4, H2O; (e) 2-methyl-3-butyn-2-ol, PdCl2(PPh3)2, CuI, TEA, DBU, THF anhydrous; (f) NaH, toluene; (g) PdCl2(PPh3)2, CuI, TEA, DBU, THF anhydrous. | |
Purity and structural analysis
The purity of intermediates and final products was confirmed by thin-layer chromatography (TLC), gas chromatography equipped with flame ionization and mass selective detector GC-FID/MS (Shimadzu GCMS-QP2010S), and by high-performance liquid chromatography HPLC-PDA-MS (APCI-ESI-dual source) (Shimadzu LCMS-2010 EV, column Kinetex C18 and PFP; 50 mm × 3.00 mm × 2.6 μm) in water/acetonitrile or water/methanol. Products were purified by crystallization and column liquid chromatography (or in the case of liquid products column chromatography only) to confirm final purity greater than 99.5%. Optical purity: 99% ee for compounds 10, 11, 12 and 13 and 98% ee for compounds 8, 9 respectively.
Mesomorphic properties
Phase transition temperatures and enthalpy data were determined by polarising optical microscopy (POM) with an OLYMPUS BX51 equipped with a Linkam hot stage THMS-600 and differential scanning calorimeter (SETARAM DSC 141) during heating/cooling cycles (rate 2 K min−1).
Selective light reflection measurements and helical twisting power calculations
Selective reflection measurements were conducted with UV-3600 Shimadzu spectrophotometer using Peltier's temperature controller.
The helical twisting power β [μm−1] was determined using a selective light reflection method19 and evaluated according to eqn (1):
| | |
β [μm−1] = (p × ee × xe)−1,
| (1) |
where
xe is the mole fraction of chiral compound and
p is the helical pitch, ee is the enantiomeric excess.
19 The helical pitch as a linear function of selectively reflected light wavelength
λmax is defined by the relationship
(2):
where
naverage is the average refractive index, approximately 1.5 for liquid crystals.
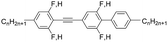
β values were determined at several different weight solutions: 5 ÷ 10 wt% in three different nematic hosts (see
Table 1) by the method described in
ref. 7b. First host mixture 1980 is based on structurally similar to the investigated in this article compounds: fluoro-substituted alkyl–alkyl phenyltolanes, described in
ref. 16, with the following properties:
TN-Iso = 106 °C, melting point
Tm < −10 °C, birefringence Δ
n = 0.31 measured at 20 °C for sodium line D, Δ
ε = 2.04 measured at 1 kHz and 20 °C. Helical twisting power values measured in this host are indicated
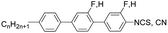
β1. The second host mixture 1816, the dielectrically positive nematic, six component material containing two- and three-ring isothiocyanates and cyanates has a nematic range
TN-Iso = 87.6 °C, melting point
Tm < −10 °C, and birefringence Δ
n = 0.22 measured at 20 °C for sodium line D. Helical twisting power values measured in this host are indicated
β2. The third host composition was the dielectrically negative nematic mixture 1754 (Δ
ε = −1.82 measured at 1 kHz and 20 °C), containing fluorinated terphenyls. Temperatures of phase transitions are the following
Tm = 16.7 °C,
TN-Iso = 114.2 °C, optical anisotropy is Δ
n = 0.25 measured at 20 °C for sodium line D. Twisting power values measured in this host are indicated
β3.
Table 1 Nematic host mixtures for helical twisting power measurements
| Indicator |
Nematic host |
Dielectric anisotropy (Δε) |
Structure |
| β1 |
1980 |
+2.5 |
 |
| β2 |
1816 |
+15.0 |
 |
| β3 |
1754 |
−1.8 |
 |
Results and discussion
Mesomorphic properties
Characterized liquid crystal compounds possess (S)-2-methylbutyl chain as a chiral source either at one or both terminal sides of molecule. In case where only one branched chain is presented, the second side of molecule is ended with carbon atom equivalent, n-pentyl alkyl chain. Temperatures and enthalpies of phase transitions are listed in Table 2. Compounds are distinguished by a broad N* phase temperature range. Additionally, homologues with two fluorine atoms (9, 11, 13) have much lower melting and clearing temperatures than compounds mono fluoro-substituted (8, 10, 12), however N* phase range is broader for difluoro homologues. This is the phenomena of 2,6-difluorophenylacetylene unit, which strongly stabilizes nematic phase. Melting temperatures below 30 °C allow to broaden the range of N* phase even up to 100 K, which is still difficult to obtain for single liquid crystal chiral compounds. It is also worth to add that BP-N* transitions were observable in DSC thermograms with its fine enthalpies. This basic property hardly ever met in liquid crystal systems, in our case was obtained only for dichiral homologues 8 and 9 (see Fig. 2).
Table 2 Phase transition temperatures and enthalpies (J mol−1) of investigated phenyltolanes. Temperatures of melting are taken from heating, while N*-BP and clearing temperatures are listed from cooling
| No. |
Temperature [°C] and enthalpy [J mol−1] of phase transition |
| Obtained from DSC. Obtained from polarizing thermomicroscope. |
| 8a |
Cr |
102.2 (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 329) 329) |
N* |
124.8 (13.1) |
BP |
126.6 (366.2) |
Iso |
| 9a |
Cr |
56.5 (17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 988) 988) |
N* |
105.1 (10.0) |
BP |
106.5 (339.9) |
Iso |
| 10b |
Cr |
89.2 (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 134) 134) |
N* |
156.7 (831.5) |
Iso |
| 11b |
Cr |
30.9 (18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 715) 715) |
N* |
133.5 (841.3) |
Iso |
| 12b |
Cr |
75.2 (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 497) 497) |
N* |
151.0 (1108) |
Iso |
| 13b |
Cr |
27.4 (24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 530) 530) |
N* |
130.8 (889) |
Iso |
 |
| | Fig. 2 (a) DSC thermogram of compound 8. (b) DSC thermogram of compound 9. | |
Selective light reflection and helical twisting power
Synthesized compounds and their mixtures in achiral nematics show selective reflection in the near infrared region 0.7 ÷ 2.5 μm (see Fig. 3). Additionally, compounds are characterized by an excellent miscibility with nematic hosts – primarily due to low enthalpies of crystalline–nematic transitions (17 ÷ 24 kJ mol−1 – Table 2) and their structural compatibility with nematic hosts, we were able to prepare even up to 20 weight% solutions. By the formulation of different concentrations, it is shown in Fig. 3, that selective reflection is still placed in NIR region. Therefore compounds should stand as perfect ingredients of cholesteric mixtures, especially when precisely fixed reflection band is needed.
 |
| | Fig. 3 Transmission spectra of thin layers of compound 9 in 1980 nematic host mixture recorded at 25 °C for three different concentrations: green: 18.5 wt%; red: 14 wt%; black: 7 wt%. | |
For all investigated compounds helical twisting power was determined every 5 K step in temperature range: 10 °C to 90 °C. β1 values were obtained for all synthesized homologues, while β2 and β3 only for dichiral compounds 8 and 9. Results for two main temperatures 20 °C and 50 °C are listed in Table 3. Fig. 4 and 5 show temperature dependence of experimental results over the whole range. Helical twisting power, as one of the parameters that informs us of chiral dopants ability to induce the helical structure in achiral liquid crystalline host, helps to categorize chiral dopants.20 It is also proven that achiral nematic medium has strong influence on helical twisting power values for chiral liquid crystalline compounds.21 We decided to select three structurally different achiral nematic hosts, to sufficiently describe synthesized phenyltolanes. Investigated compounds are characterized by moderate values of the helical twisting power indicator. It is clearly seen that compounds 8 and 9 with two chiral chains have much higher values of β1 than single-chiral homologues 10–13 (see Fig. 4). Results for 1816 (β2) and 1754 (β3) do not bring significant difference. Twisting power is higher for compound 9 than for compounds 8 in three different hosts. We can also find, that twisting power values decrease with temperature for 1980 and 1816 hosts (β1 and β2), whereas for dielectrically negative nematic 1754 host, β3 values increase, especially at lower temperature regions.
Table 3 Comparison of helical twisting power β values for compounds 8 and 9 in different nematic achiral hosts, obtained at 20 °C and 50 °C
| Helical twisting power β [μm−1] |
|
Compound number |
| 8 |
9 |
| β1 |
20 °C |
9.17 |
9.53 |
| 50 °C |
8.68 |
8.97 |
| β2 |
20 °C |
11.10 |
11.98 |
| 50 °C |
11.04 |
11.64 |
| β3 |
20 °C |
10.19 |
12.08 |
| 50 °C |
10.33 |
12.01 |
 |
| | Fig. 4 Temperature dependence of β1 values for compounds 8–13. | |
 |
| | Fig. 5 Temperature dependence of helical twisting power values β2 (red symbols) and β3 (blue symbols) for compounds 8 and 9. | |
Summary
We have synthesized and evaluated physical properties of new fluorinated phenyltolane based chiral liquid crystal materials with (S)-2-methylbutyl chains. Investigated compounds are characterized by a broad temperature range of N* phase, very low melting temperatures and easy to achieve selective reflection in the near infrared region. That makes them excellent candidates for a cholesteric mixture formulation, designed for example for energy-saving exploitation. High stability of the chiral nematic mesophase originates mainly from the rigid core which consists of 2,6-difluorophenyl acetylene structural substructure, investigated in detail.15–18 Its phenomenon leads to a perfect miscibility of investigated compounds in different achiral nematics. Moderate values of twisting power come from the origin of chiral source used here, but its accessibility should be found as a huge advantage.
Acknowledgements
Work was supported by the PBS 23-651 grant and Military University of Technology grant 08-794.
Notes and references
- H. Q. Xianyu, S. Faris and G. P. Crawford, Appl. Opt., 2004, 43, 5006–5015 CrossRef CAS PubMed.
- J. Xiang, Y. N. Li, Q. Li, D. A. Paterson, J. M. D. Storey, C. T. Imrie and O. D. Lavrentovich, Adv. Mater., 2015, 27, 3014–3018 CrossRef CAS PubMed.
- M. H. Lu, J. Appl. Phys., 1997, 81, 1063–1066 CrossRef CAS.
- K. H. Kim, D. H. Song, Z. G. Shen, B. W. Park, K. H. Park, J. H. Lee and T. H. Yoon, Opt. Express, 2011, 19, 10174–10179 CAS.
- T. Manabe, K. Sonoyama, Y. Takanishi, K. Ishikawa and H. Takezoe, J. Mater. Chem., 2008, 18, 3040–3043 RSC.
-
(a) O. Chojnowska and R. Dabrowski, Photonics Lett. Pol., 2012, 4, 81–83 CAS;
(b) Y. Chen and S. T. Wu, J. Appl. Polym. Sci., 2014, 131 Search PubMed.
-
(a) O. Yokokoji, M. Oiwa, T. Koike and S. Inoue, Liq. Cryst., 2008, 35, 995–1003 CrossRef CAS;
(b) P. Kula, J. Herman and O. Chojnowska, Liq. Cryst., 2013, 40, 83–90 CrossRef CAS;
(c) M. Itoh, H. Hegi, K. Marumo, K. Sakajiri, S. M. Kang, M. Tokita and J. Watanabe, Liq. Cryst., 2013, 40, 339–344 CrossRef CAS;
(d) R. Bitar, G. Agez and M. Mitov, Soft Matter, 2011, 7, 8198–8206 RSC;
(e) M. Moriyama, S. Song, H. Matsuda and N. Tamaoki, J. Mater. Chem., 2001, 11, 1003–1010 RSC;
(f) G. Shanker and C. V. Yelamaggad, New J. Chem., 2012, 36, 918–926 RSC;
(g) M. E. McConney, T. J. White, V. P. Tondiglia, L. V. Natarajan, D. K. Yang and T. J. Bunning, Soft Matter, 2012, 8, 318–323 RSC;
(h) L. Rossi, S. Sacanna and K. P. Velikov, Soft Matter, 2011, 7, 64–67 RSC;
(i) B. R. Li, W. L. He, L. Wang, X. Xiao and H. Yang, Soft Matter, 2013, 9, 1172–1177 RSC.
- K. Oki, M. Ichihashi and H. Yamada, Infrared light reflector, infrared light reflecting laminated glass, and laminated glass and laminate have cholesteric liquid crystal layers, US Pat., US20120094118 A1, 2010.
- Y. Matsumoto, K. Tamura, K. Harai, Y. Fujino, K. Kawabata and K. Arakawa, SID Int. Symp. Dig. Tech. Pap., 2009, 40, 1559–1562, DOI:10.1889/1.3256612.
- T. Tasaka, M. Kimura, M. Nakajima and T. Hashizume, Cholesteric liquid crystal mixture, film, ir reflection plate, laminate, and laminated glass, US Pat., US20150010761 A1, 2014.
-
(a) H. Khandelwal, R. C. G. M. Loonen, J. L. M. Hensen, A. P. H. J. Schenning and M. G. Debije, J. Mater. Chem. A, 2014, 2, 14622–14627 RSC;
(b) H. Khandelwal, R. C. G. M. Loonen, J. L. M. Hensen, M. G. Debije and A. P. H. J. Schenning, Sci. Rep., 2015, 5, 11773 CrossRef PubMed;
(c) H. Q. Xianyu, S. Faris and G. P. Crawford, Appl. Opt., 2004, 43, 5006–5015 CrossRef CAS PubMed;
(d) K. H. Kim, H. J. Jin, K. H. Park, J. H. Lee, J. C. Kim and T. H. Yoon, Opt. Express, 2010, 18, 16745–16750 CrossRef CAS PubMed.
- M. Mitov, Adv. Mater., 2012, 24, 6260–6276 CrossRef CAS PubMed.
- R. Dabrowski, P. Kula and J. Herman, Crystals, 2013, 3, 443–482 CrossRef CAS.
-
(a) R. Eelkema and B. L. Feringa, Org. Biomol. Chem., 2006, 4, 3729–3745 RSC;
(b) Handbook of Liquid Crystals, ed. D. Demus, J. Goodby, G. W. Gray, H.-W. Spiess and V. Vill, Wiley-VCH, Weinheim, 1998, vol. 1–3 Search PubMed;
(c) J. W. Goodby, Mol. Cryst. Liq. Cryst., 1997, 292, 245–263 CrossRef;
(d) I. Nishiyama, Chem. Rec., 2009, 9, 340–355 CrossRef CAS PubMed.
-
(a) R. Buchecker, G. Marck and M. Schadt, Mol. Cryst. Liq. Cryst. Sci. Technol., Sect. A, 1995, 260, 93–105 CrossRef CAS;
(b) F. Seils and M. Schadt, Mol. Cryst. Liq. Cryst. Sci. Technol., Sect. A, 1995, 260, 127–138 CrossRef CAS.
- P. Kula, A. Aptacy, J. Herman, W. Wojciak and S. Urban, Liq. Cryst., 2013, 40, 482–491 CrossRef CAS.
- J. Herman and P. Kula, Tetrahedron Lett., 2013, 54, 3621–3623 CrossRef CAS.
- S. Liang, Z. Zhi-Yong, Y. Hong-Jun, D. Zhi-Qun, W. Ben-Mei, X. Li and P. Zeng-Hui, Chin. J. Liq. Cryst. Disp., 2010, 6, 784–791 Search PubMed.
- Ch. Mauguin, Bull. Soc. Fr. Mineral. Cristallogr., 1911, 34, 71–117 Search PubMed.
-
(a) W. Rejmer, K. Czuprynski and M. Zurowska, Mol. Cryst. Liq. Cryst., 2011, 547, 3–9 CAS;
(b) H. G. Kuball, H. Bruning, T. Muller, O. Turk and A. Schonhofer, J. Mater. Chem., 1995, 5, 2167–2174 RSC.
- W. Rejmer, K. Czuprynski and W. Drzewinski, Mol. Cryst. Liq. Cryst., 2011, 547, 33–38 CAS.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–) group.13 Highly birefringent liquid crystals are often difficult to synthesize, moreover solubility of such structures is also usually poor. For these reasons, the fabrication of novel cholesteric liquid crystals with increased birefringence, hence broad bandgap is challenging. Typical commercially available chiral compounds show rather poor solubility in the liquid crystal host (several wt%), mainly because of structural incompatibility with widely used nematic hosts. Additionally, for a vast majority of cholesteric liquid crystals used nowadays Δn is less than 0.3.14 In this work we focused on synthesis of new class of liquid crystal materials based on phenyltolane core, which assures increased birefringence Δn > 0.3. Lateral fluorine substitution, 2,6-difluorophenylacetylene unit in particular brings unique and valuable nematic stability,15–17 which in connection with a chiral centre broadens significantly the cholesteric (N*) phase range, even up to 100 K. As a chiral source (S)-2-methylbutyl alkyl chain was chosen, mainly because it's easily accessible. Although the selected chiral chain, due to lack of strong polar substituents in vicinity to asymmetric carbon, does not provide high helical twisting power (β < 20 μm−1), still great solubility and miscibility with various achiral hosts make these kind of structures promising materials. Compounds were designed to show structural similarity to widely used nematic achiral hosts, in that way the solubility and interactions with hosts could be enhanced. As a result, solutions of different concentrations of chiral compound (5 ÷ 20 wt%) were composed, which shows reflection in the near infrared region.
C–) group.13 Highly birefringent liquid crystals are often difficult to synthesize, moreover solubility of such structures is also usually poor. For these reasons, the fabrication of novel cholesteric liquid crystals with increased birefringence, hence broad bandgap is challenging. Typical commercially available chiral compounds show rather poor solubility in the liquid crystal host (several wt%), mainly because of structural incompatibility with widely used nematic hosts. Additionally, for a vast majority of cholesteric liquid crystals used nowadays Δn is less than 0.3.14 In this work we focused on synthesis of new class of liquid crystal materials based on phenyltolane core, which assures increased birefringence Δn > 0.3. Lateral fluorine substitution, 2,6-difluorophenylacetylene unit in particular brings unique and valuable nematic stability,15–17 which in connection with a chiral centre broadens significantly the cholesteric (N*) phase range, even up to 100 K. As a chiral source (S)-2-methylbutyl alkyl chain was chosen, mainly because it's easily accessible. Although the selected chiral chain, due to lack of strong polar substituents in vicinity to asymmetric carbon, does not provide high helical twisting power (β < 20 μm−1), still great solubility and miscibility with various achiral hosts make these kind of structures promising materials. Compounds were designed to show structural similarity to widely used nematic achiral hosts, in that way the solubility and interactions with hosts could be enhanced. As a result, solutions of different concentrations of chiral compound (5 ÷ 20 wt%) were composed, which shows reflection in the near infrared region.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 329)
329)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 988)
988)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 134)
134)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 715)
715)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 497)
497)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 530)
530)







