A forest of SiO2 nanowires covered by a TiO2 thin film for an efficient photocatalytic water treatment
Abstract
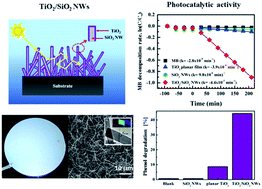
We propose a highly disordered and randomly oriented array (forest) of SiO2 nanowires (NWs) coated by a thin film of TiO2 as a performing photocatalytic material for water treatment applications. The SiO2 NWs with a length of tens of microns were obtained via thermal oxidation of Si NWs, grown by plasma enhanced chemical vapour deposition (PECVD), and covered by a nanometric TiO2 film deposited by atomic layer deposition (ALD). The remarkable photocatalytic performance of the TiO2/SiO2 NWs was demonstrated by the degradation of methylene blue and phenols in water. The enhanced photocatalytic activity in the overlaying TiO2 film is attributed to the synergetic effect of the morphology and optical properties of the SiO2 NWs. The fabrication methods based on the well-established Si technology make the proposed materials a promising strategy for water treatment.

 Please wait while we load your content...
Please wait while we load your content...