Synergistic effect of phosphorus–nitrogen and silicon-containing chain extenders on the mechanical properties, flame retardancy and thermal degradation behavior of waterborne polyurethane†
Abstract
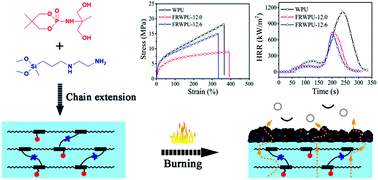
With the aim to keep a balance between the flame retardancy and thermal stability as well as mechanical properties of waterborne polyurethane (WPU), a novel phosphorus–nitrogen–silicon containing flame retardant WPU (FRWPU) was synthesized by conjugating with a cyclic phosphoramidate lateral group bearing diol (named as PNMPD) and silane coupling agent KH-602 in the chain-extension and post-chain extension process, respectively. Significant enhancement in tensile strength (6.1 MPa) is obtained with the combined covalent incorporation of PNMPD and KH-602 rather than using PNMPD alone. A limiting oxygen index (LOI) value of 27.7% and a UL-94 vertical burning V-0 rating is achieved for FRWPU-12.6 with 12 wt% PNMPD and 60% post-chain extension ratio by KH-602, while the peak heat release rate (PHRR), total heat release (THR), peak smoke produce rate (SPR) and total smoke production (TSP) characterized by a cone calorimeter (CC) markedly reduce by 36.0%, 42.9%, 40.1% and 35.4%, respectively, compared to those of pure WPU, which is more efficient in flame retardancy than FRWPU-12.0 merely loaded with 12 wt% PNMPD. Meanwhile, the thermal degradation behaviors and flame-retardant mechanism of FRWPU were consistently confirmed by thermogravimetric analysis (TGA), scanning electron microscopy (SEM), thermogravimetry-Fourier transform infrared (TG-FTIR) spectroscopy, X-ray photoelectron spectroscopy (XPS) and Raman spectroscopy. All results indicate that the interactions between phosphorus and silicon elements in the condensed phase are mainly responsible for the dramatically reduced fire hazards, which inhibits the heat and flammable gas release and facilitates the formation of a more thermally stable graphitized char layer consisting of –P(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–O–Si– structures.
O)–O–Si– structures.

 Please wait while we load your content...
Please wait while we load your content...