Promotion effects of Pd on tungsten carbide catalysts: physiochemical properties and cellulose conversion performance†
Abstract
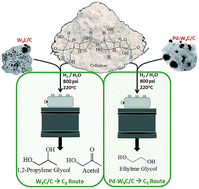
A multi-functional catalyst, which is able to perform both retro-aldol reactions followed by hydrogenation, is required to convert cellulose into value-added ethylene glycol (EG) in a one-pot reaction. Herein, we show the results of a catalyst comprising Pd-promoted tungsten carbide supported on activated carbon. Pd acts to hinder carbon deposition onto the catalyst surface during the carburization process, through the inhibition of methane decomposition species on the catalyst surface. XPS and TEM analysis show a cleaner surface which improves the W/C ratio. The enhanced interaction between Pd and W2C, results in higher cellulose conversions, and higher ethylene glycol (EG) yields when compared to non-promoted or Ni promoted tungsten carbide catalysts reported elsewhere. XAS, HR-TEM and SI-XEDS analysis showed Pd nanoparticles are well dispersed on the surface and in contact with W2C. Despite higher carburization temperatures, Pd-promoted tungsten carbide catalysts enhance the catalytic properties of the one-pot cellulose conversion reaction towards EG production when compared to Ni-promoted catalysts under the same catalytic reaction conditions.

 Please wait while we load your content...
Please wait while we load your content...