The enhanced CO gas sensing performance of Pd/SnO2 hollow sphere sensors under hydrothermal conditions
Qingji Wang†
a,
Xu Li†c,
Fangmeng Liub,
Chang Liub,
Tan Sud,
Jun Lin*a,
Peng Sunb,
Yanfeng Sunb,
Fengmin Liub and
Geyu Lu*b
aCollege of Instrumentation and Electrical Engineering, Jilin University, Ximinzhu Street, Changchun, 130061, China. E-mail: wangqingji@jlu.edu.cn
bState Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, 2699 Qianjin Street, Changchun 130012, China. E-mail: lugy@jlu.edu.cn; Fax: +86-431-85167808; Tel: +86-431-85167808
cDivision of Chemistry and Biological Chemistry, School of Physical and Mathematical Sciences, Nanyang Technological University, 21 Nanyang Link, 637371, Singapore
dInstitute of Theoretical Chemistry, Jilin University, Changchun 130021, China
First published on 15th August 2016
Abstract
Pd-doped SnO2 hollow spheres were synthesized via a facile one-step hydrothermal route. Utilized as the materials in sensors, the 3.0 wt% Pd-doped SnO2 demonstrated more excellent gas-sensing properties towards CO than 1.5 wt% and 4.5 wt% Pd-doped SnO2. Compared with the SnO2 hollow spheres gas sensor, the optimum operating temperature of the Pd-doped SnO2 hollow spheres gas sensor dropped to 200 °C from 250 °C; the response value to 100 ppm CO was raised to 14.7 from 2.5 accordingly. Furthermore, the response and recovery times of the 3.0 wt% Pd-doped SnO2 sensor are 5 s and 92 s, respectively, to 100 ppm CO at 200 °C. It is believed that its enhanced gas-sensing performances are derived from the synergistic interactions between the dispersed Pd and the characteristic configuration of the SnO2 hollow sphere. In addition, theoretical calculations have also been performed with periodic slab models by using density functional theory, which explain well our experimental phenomenon.
1. Introduction
Various oxide semiconductor based gas sensors have been used to detect harmful and toxic gases, such as SnO2,1 ZnO,2 In2O3,3 Fe2O3,4 Co3O4,5 TiO2,6 NiO,7 WO3,8 MnO2,9 V2O5.10 Oxide semiconductor based gas sensors have attracted much interest to their appealing features including low cost, environmental friendliness, high sensitivity and fast response and recovery time.11,12 Among them, SnO2 as the most representative sensor material has been widely reported with a number of methods for CO detection. The previous works are shown in Table 1. Considering their applications, SnO2-based sensing materials have been successfully prepared in the form of various structures including cubes,13 spheres,14 fibers15 and tubes16 etc. In particular, in contrast to conventional solid structured materials, a hollow structure can provide high surface area, which is of great significance to gas diffusion and fast mass transport in sensor materials, thus leading to an improved sensing performance.17| Target gas | Methods | Morphologies | Temp. | Concentration | S | Ref. |
|---|---|---|---|---|---|---|
| a S = Ra/Rg. | ||||||
| CO | Vapor–liquid–solid growth | Pd/SnO2 nanoparticles | 400 °C | 10 ppm | 2.2 | 25 |
| CO | Chemical vapor deposition | Pd-doped SnO2 nanorod | 350 °C | 1000 ppm | 7 | 26 |
| CO | Sol–gel | FePd/SnO2 nanoparticles | 350 °C | 2000 ppm | 23 | 27 |
| CO | Thermal evaporation | Pd/SnO2 nanowires | 400 °C | 100 ppm | 5 | 28 |
| CO | Hydrothermal | Co/Sn oxide flake-flower | 200 °C | 100 ppm | 6 | 29 |
| CO | Pulsed laser deposition | SnO2/CuO nanoparticles | 180 °C | 100 ppm | 9.5 | 30 |
| CO | Hydrothermal | Pd/SnO2 hollow sphere | 200 °C | 100 ppm | 14.7 | This work |
Hollow structure has attracted much attention due to their outstanding features of low mass density and tailored structure with less agglomerated configurations.18 The special hollow interior with a porous surface structure not only increases the specific surface area of the material, but also gives rise to intriguing properties resulting from the electron distribution on the surface of the novel nanostructures.19 Benefitting from the above merits, hollow structure present numerous application prospects in a wide range of fields such as gas sensors, photocatalysts, drug delivery, supercapacitors, and lithium-ion batteries.20 Template-engaged and self-templating methods have been achieved to synthesize hollow structures, which enhance the gas sensors performance for target gases. It has been demonstrated that self-templating approaches, such as Ostwald ripening, could be exploited to produce the anticipated hollow structures simply.7 Furthermore, with the development of the semiconductor gas sensors, suitable additives such as noble metals (Pd, Au, Pt)21–23 have been employed, serving as sensitizers or promoters to improve the sensing properties dramatically. Pd-doped on SnO2 has been widely reported owning to an improvement of gas sensor properties24 because Pd is a typical material of electronic sensitization.
Herein, Pd-doped SnO2 hollow sphere with different amount of Pd were prepared by a facile one-step hydrothermal method. Followed by subsequent annealing process, the CO gas sensing of as-prepared sample were investigated. Compared with un-doped SnO2, the introduction of Pd will be helpful in promoting the overall chemical and electronic properties. Thus, the as-synthesized Pd-doped SnO2 manifest a high response with a fast response towards CO when they are evaluated as sensing materials. Moreover, the theoretical calculations are performed with periodic slab models by using density functional theory to investigate the sensing mechanism of CO oxidation on un-doped and Pd-doped SnO2.
2. Experimental
2.1 Synthesis and characterization of the sensing materials
All the reagents in the experiment were analytical-grade purity (Beijing Chemicals Co. Ltd.) and used as received without any further purification. In our experiment, Pd-doped SnO2 hollow sphere were synthesized by a facile hydrothermal route. Also K2SnO3·3H2O and PdCl2 were used as tin and palladium sources, respectively. In a typical synthesis process, K2SnO3·3H2O (42 mg), and urea (0.18 g) were added into a solution of ethanol and water (15 mL, 15 mL) with vigorous stirring for 10 min. Then certain amount of as-prepared PdCl2 were added into above mixture solutions respectively, thus, the corresponding mass ratio between element Pd and element Sn is 1.5 wt%, 3.0 wt%, 4.5 wt% respectively. After stirred for 10 min to form a homogeneous solution, the above mixture was transferred into 45 mL Teflon-lined autoclave, which was sealed and kept at 150 °C for 24 h. After the hydrothermal procedure, the resultant precipitates were centrifuged and washed with deionized water and absolute ethanol several times before drying at 80 °C for 12 h. Finally, the obtained powder was calcined at 500 °C for 2 h in muffle furnace. A series of 0.0, 1.5, 3.0, and 4.5 wt% Pd-doped SnO2 hollow sphere were synthesized.Powder X-ray diffraction (XRD) patterns were performed on a Rigaku D/max-2550 X-ray diffractometer with Cu-Ka radiation (λ = 0.1541 nm) operating at 40 kV in the range of 20–80 degree. Field emission scanning electron microscopy (FESEM) images were recorded on a JEOL JSM-7500F microscope with an acceleration voltage of 15 kV. Transmission electron microscopic (TEM) were recorded on a JEOL JEM-3010 transmission electron microscope with an acceleration voltage of 200 kV.
2.2 Fabrication and measurement of gas sensors
The gas sensors fabrication process based on as-prepared sensing materials were described detailed in our previous works,31 and sensors devices were sintered at 400 °C for 2 h to improve its stability and performance. The gas sensing performance of the gas sensors were investigated using a static system under laboratory conditions (40% relative humidity, 25 °C). The sensors were put into an airtight chamber (50 L in volume) purged with pure air, and then a given amount of test gases was injected into the airtight chamber using a microsyringe for the measurement of the gas-sensing performance. The sensor was alternately placed into closed test chambers with pure air or target gas. The response (Ra/Rg) of the sensor was defined as the ratio of the resistances of the sensors in air (Ra) to that in tested gases (Rg). The response and recovery times were defined as the times taken by the sensor to achieve 90% of the total resistance changes.2.3 Density functional theory (DFT) calculations
The periodic slab models were used to calculate SnO2 (110) surface, which is the most thermostatically stable and widely studied in theoretical study.32 In this work, we adopted the four O(Sn2O2)O trilayers, including five and six-fold coordinated Sn, two-fold coordinated bridging O, and three-fold coordinated in plane O, respectively. The slab was separated by a vacuum spacing of 10 Å. The bottom two trilayers were fixed in lattice position. Basis on the experimental knowledge, the reduced SnO2 (110) surface can adsorbed oxygen species O22−.33 Initial configuration of CO on SnO2 (110) surface with pre-adsorbed O22− species was considered in our calculations. The substitution model of Pd-doped on the SnO2 (110) surface was constructed: Pd substituted five-fold coordinated Sn.The density functional theory (DFT) was carried out the semi-core pseudopots34 with generalized gradient approximation (GGA) of Perdew–Burke–Ernzerhof (PBE) method to treat the exchange and correlation potentials.35 The double numerical basis set (DND basis set) was employed. All of transition states were determined by the linear synchronous transit (LST) and quadratic synchronous transit (QST) methods. The convergence criteria for optimizing geometry for energy, force and displacement were 2 × 10−5 ha, 4 × 10−3 ha, and 5 × 10−3 ha, respectively. The width of the Fermi smearing of the Kohn–Sham states was set to kBT = 0.005 ha. The reciprocal space was sampled using a (2 × 2 × 1) k-point grid generated automatically using the Monkhorst–Pack method.36 All of calculations were performed using DMol3 code in Materials Studio.37
In this work, the absorption energy was computed by the energy difference of gas-phase guest molecule and surface species (slab) as eqn (1). The reaction barrier was computed by the energy difference of the transition state (TS) and reactant as eqn (2). The reaction energy was computed by the energy difference of product and reactant as eqn (3).
| Eads = Egas molecule/slab − Egas molecule − Eslab | (1) |
| Ebarrier = Ereactant − ETS | (2) |
| Ereaction = Eproduct − Ereactant | (3) |
With this definition, negative adsorption energy corresponds to an energetically favorable adsorption site on the surface and more negative values correspond to stronger adsorption interaction. The reaction pathway with low reaction barrier is kinetically favorable and the more negative value of reaction energy corresponds to the thermodynamically favorable reaction.
3. Results and discussion
3.1 Materials characterization
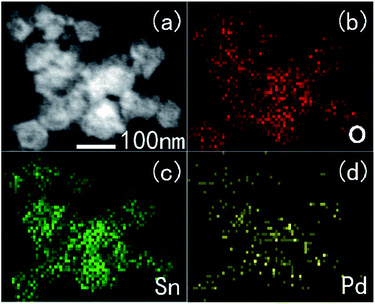
The typical X-ray diffraction (XRD) patterns of the prepared un-doped and Pd-doped SnO2 are shown in Fig. 1. All the diffraction peaks were coincident with tetragonal structure of SnO2 with (JCPDS file no. 77-451). That was, they could be indexed as SnO2 with lattice constants of a = 0.47 nm and c = 0.32 nm. The prominent positions of the (110), (101) and (211) diffraction peaks from Pd-doped SnO2 hollow sphere make no significant shift to higher angles compared to the un-doped SnO2. It is also observed that full width at half maxima (FWHM) of the diffraction peaks are almost the same, these make clear that no significant differences existed in the crystalline degree with the increasing doped amounts of Pd. No peaks of Pd were observed because of its small doped amounts.The morphology and microstructures of the un-doped SnO2 and Pd-doped SnO2 were characterized using SEM, as shown Fig. 2. A number of sphere-like architectures could be clearly observed. No other morphologies were detected, indicating a high yield of these microstructures. The SEM image of as-prepared samples, seen in the inset of Fig. 2, shows the sphere-like architectures with a well hollows structure before they were calcined at 500 °C. The hollow sphere structures are showed in the square. Furthermore TEM image of 3.0 wt% Pd-doped SnO2 and the corresponding elemental mapping images (Fig. 3) confirmed that Pd show uniform distribution in hollow sphere SnO2, which further proved that Pd were well disperse in SnO2 in our experimental conditions.
 | ||
| Fig. 2 SEM images of Pd-doped hollow sphere SnO2 (a) 0 wt%, (b) 1.5 wt%, (c) 3.0 wt% and (d) 4.5 wt%. | ||
3.2 Carbon monoxide gas-sensing properties
Using prepared sensing materials, four gas sensors were fabricated and their CO gas sensing performances were investigated. In order to determine the optimum operating temperature, the responses of the Pd-doped hollow sphere SnO2 gas sensor and un-doped SnO2 gas sensor to 100 ppm CO were measured at different operating temperatures. Fig. 4a shows the responses as a function of operating temperature from 175 to 275 °C. It is obvious that the response of all of four sensors varied with operating temperature. As the operating temperature increased, the responses of the Pd-doped hollow sphere SnO2 gas sensors increased at first and reached the maximum at 200 °C, followed by a decrease gradually. While the un-doped SnO2 gas sensor reached the maximum response at 250 °C. It is obvious that the optimal temperature was reduced than the un-doped SnO2 gas sensor. Because of the catalysis of the Pd nanoparticles, the activation energy between the target gas and adsorption oxygen was decreased. Thus the optimal temperature was reduced to 200 °C from 250 °C. The maximum responses of four gas sensors are 2.5, 4.9, 14.7 and 5.4 at their optimum operating temperature respectively. It can be seen that the sensor based on 3.0 wt% Pd-doped SnO2 displayed the most notably enhanced response to carbon monoxide compared with that based on other samples. The highest response of the sensor based on 3.0 wt% Pd-doped SnO2 was much more 5 times higher than that of un-doped SnO2 to 100 ppm carbon monoxide at their optimal operating temperature separately. | ||
| Fig. 4 Responses of the sensors base on as-prepared samples vs. operating temperature to 100 ppm CO (a) and their response times (b). | ||
Rapid response and recovery are necessary in practical application. The sensors were tested to 100 ppm CO at different working temperature respectively. From the Fig. 4b, we can see that the response time of four sensors are faster at higher temperature than those in lower temperature. The response times of Pd-doped SnO2 sensors are all faster than that of un-doped sensor, but only the recovery time of 1.5 wt% sensor is faster than that of un-doped sensor. The response time and recovery time of 3.0 wt% sensor is slower than those of 1.5 wt% and 4.5 wt% sensors probably due to the differences of electron depleted layer, but the 3.0 wt% sensors perform the best enhancement among sensors of different Pd doped amount. The following studies about the CO sensing properties of sensors were operated at their optimal temperature.
Further gas-sensing properties of the sensors to different concentrations of carbon monoxide were investigated when sensors working at theirs optimal operating temperatures. Fig. 5 shows the typical response curves of the Pd-doped hollow sphere SnO2 gas sensor and the un-doped SnO2 gas sensor with an increasing concentration of CO at their optimal temperature. From 10 to 200 ppm, the responses of the Pd-doped hollow sphere SnO2 gas sensor linearly increased. In particular, the discrepancy in response values and slope of the curve indicated that the sensing performance was highly dependent upon the Pd-doped amount. The response of Pd-doped hollow sphere SnO2 gas sensor was much higher than the un-doped SnO2 gas sensor in the concentration range considered here. We can see that the sensor based on 3.0 wt% Pd-doped SnO2 exhibited higher response to carbon monoxide at various concentrations compared with that based on 1.5 wt% and 4.5 wt% Pd-doped SnO2. This result can be interpreted in terms of the electric interaction between Pd and SnO2, for which produced electron depleted layer on the surface of SnO2. However, doped more than 4.5 wt% decreased the resistance, it may be caused by the agglomeration of Pd particles, thus may impede the enhancement of CO gas sensing properties.
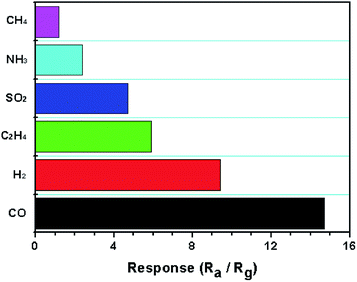
Moreover, the four periods of repetitive test are shown in Fig. 6 to 100 ppm CO at theirs optimal operating temperatures, indicating the good repeatability and stability of the sensor. It can be seen that the resistance of the sensor decreased when interact with carbon monoxide, and then reached a steady state in 2 min. Soon afterwards, the sensor was transferred into air to recover and this process takes 4 min respectively. The selectivity of the sensor based on 3.0 wt% Pt/SnO2 was shown in Fig. 7 toward 100 ppm various gas at 200 °C. We can see that it perform the better selectivity for CH4. Our study shows that both morphology of the oxides and catalytic additives can enhance the gas sensing performance to a better degree. In order to understand the sensing mechanism of CO oxidation, density functional theory (DFT) was employed to calculate absorption energy, reaction barrier and energy (kcal mol−1) on different pathway of CO oxidation on un-doped and Pd-doped SnO2 (110) surface.
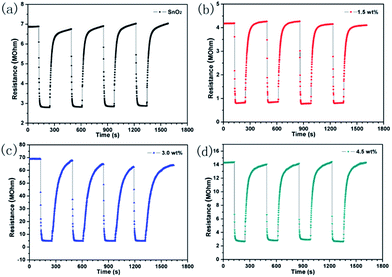 | ||
| Fig. 6 Transients curve of sensors base on as-prepared samples at 200 °C to 100 ppm CO (a) 0 wt%, (b) 1.5 wt%, (c) 3.0 wt% and (d) 4.5 wt%. | ||
3.3 Mechanism of CO oxidation
As shown in Fig. 8, CO oxidation on SnO2 (110) surface (pathway I) and the Pd-doped SnO2 (110) surface (pathway II) are proposed. In the case of reaction pathway I, O2 molecule (blue atoms) is adsorbed on the reduced SnO2 (110) surface becoming a peroxide ion: the O–O bond (1.48 Å) is cleaved, one of them occupied the lattice oxygen position to be pre-adsorbed oxygen species of O22− (Fig. 8a). The adsorption energy is calculated to be −32.1 kcal mol−1 (Table 1), which is close to the previously theoretical study.38 This process is a strongly chemical adsorption. Then, CO molecule is adsorbed on SnO2 (110) surface to be a stable geometry with the adsorption energy is −6.4 kcal mol−1, indicating that a weak interaction corresponds to be a physical absorption. The following step is oxidation reaction involving a transition state (Fig. 8b) within the bond distance of O–O, O–C and C–Sn are 1.42 Å, 2.27 Å and 2.47 Å, respectively. As shown in Fig. 8c, CO molecule reacts with the lattice oxygen and form an intermediate CO32− species. This oxidation step needs to overcome a reaction barrier of 21.6 kcal mol−1 and the reaction energy is −7.8 kcal (see Table 2). Subsequently, the formed CO32− species is adsorbed on the five-fold coordinated Sn, the bond distance of C–Sn is 2.26 Å. Then CO32− species is converted into CO2 and perfect SnO2 (110) surface is regenerated via a transition state (Fig. 8d), the O–O bond (1.74 Å) is break and the C–O bond (1.31 Å) is formed. This step should overcome a reaction barrier 12.4 kcal mol−1, and reaction energy is −82.5 kcal mol−1, indicating that is a drastically exothermic reaction. It is worth mentioning that, the second step has lower reaction barrier and more negative value of reaction energy than that in first step (Table 2). This result indicates that the second step corresponds to the kinetically and thermodynamically favorable reaction on the surface. Finally, a weak interaction between CO2 molecule and SnO2 (110) surface with the adsorption energy is −9.5 kcal mol−1 and the O–Sn bond distance is 2.66 Å, the CO2 species would be released as free molecule in room temperature. | ||
| Fig. 8 CO oxidation on SnO2 (110) surface (pathway I) and Pd-doped SnO2 (110) surface (pathway II). Blue atoms are the pre-adsorbed O22− species on the reduced SnO2 (110) surface. | ||
| Reaction | Eads-gas molecule | Ebarrier | Ereaction | |
|---|---|---|---|---|
| I | O2(g) + SnO2(d) → O22−/SnO2 | −32.1 | ||
| CO(g) + O22−/SnO2 → CO32−/SnO2 | −6.4 | 21.6 | −7.8 | |
| CO32−/SnO2 → CO2(g) + SnO2 | −9.5 | 12.4 | −82.5 | |
| II | O2(g) + Pd–SnO2(d) → O22−/Pd–SnO2 | −25.5 | ||
| CO(g) + O22−/Pd–SnO2 → CO32−(Int)/Pd–SnO2 | −4.3 | 15.0 | −36.6 | |
| CO32−(Int)/Pd–SnO2 → CO2(g) + Pd–SnO2 | −7.0 | 11.5 | −43.9 |
The Pd-doped SnO2 (110) material, in which Pd atom is incorporated into the lattice as substitutional point defects have been investigated by DFT calculation in literature. Xue et al. investigated that Pd substituted five-fold coordinated Sn forms a stable doped structure.39Robina et al. through a theoretical study found that Pd clusters are more stable on reduced SnO2 (110) surface.40 In this work, Pd-doped on SnO2 (110) surface is modeled through substituted a Pd atom for a five-fold coordinated Sn in the surface slab model, as shown in Fig. 8. Based on the same mechanism, in pathway II, O2 molecule is adsorbed on the doped Pd-doped SnO2 (110) surface with the adsorption energy is −25.5 kcal mol−1, which is weaker than that on SnO2 (110) surface (Table 1). The presence of CO molecule on the doped Pd-doped SnO2 (110) surface would also give a weak physical absorption (−4.3 kcal mol−1). The optimized geometry of adsorbed CO is shown in Fig. 8f. Then adsorbed CO molecules with O22− species transform into CO32− species via a transition state (Fig. 8g). This transition state with the bond distance of O–O, O–C and C–Sn are 1.35 Å, 2.45 Å and 2.58 Å, respectively, which geometry is similar with that on un-doped SnO2 (110) surface. However, this oxidation reaction is a strongly endothermic reaction. The reaction barrier and energy of pathway II are 15.0 and −36.6 kcal mol−1, respectively (Table 2), which is much lower than that in the pathway I, indicating that the CO oxidation to produce the intermediate CO32− species is kinetically and thermodynamically more efficient on doped Pd-doped SnO2 (110) than that on SnO2 (110) surface. The following step undergoes a transition state (Fig. 8i), the intermediate CO32− species produces CO2 molecule and feedbacks O atom to vacancy of lattice to attain the prefect Pd-doped SnO2 (110) surface. As a result, the calculated reaction barrier and energy are 11.5 and −43.9 kcal mol−1, respectively. In final product, the adsorbed CO2 molecule on Pd-doped SnO2 (110) surface with the adsorption energy is −7.0 kcal mol−1 and the O–Sn bond distance is 2.60 Å.
In our calculation results, the un-doped SnO2 (110) surface is beneficial for adsorbing O2 molecule (Eads = −32.1 kcal mol−1 in Table 2). However, for the CO and CO2 molecules, there is no big difference on un-doped and Pd-doped on SnO2 (110), the adsorption energies are closed. For the overall reaction pathways of I and II, the rate-limiting step is the CO oxidation step, requiring more activation energy and leading to a stable intermediate CO32− species. However, the pathway II with the lower reaction barrier and more negative reaction energy at rate-limiting step has a significant advantage over pathway I, which indicates that CO oxidation is easily and fast occurred on the Pd-doped SnO2 (110) surface. These results are agreed well with the experimental fact that Pd-doped on SnO2 material can improve gas-sensing properties. In addition, our calculation results will provide further understanding of CO oxidation process for gas-sensing, which is important to explain the experimental phenomenon at molecular level.
4. Conclusions
In conclusion, Pd-doped SnO2, which combined the advantages of hollow structure and catalysis of noble metal Pd, were synthesized by one-step facile hydrothermal technique. Pd nanoparticles with small size were successfully disperse on the surface of the hollow sphere SnO2, which are composed from SnO2 nanoparticles. Compared with the un-doped SnO2 gas sensor, the Pd-doped SnO2 gas sensor owned enhanced CO sensing properties, such as high response and rapid response (14.7 and 5 s to 100 ppm CO). These excellent results can be attributed to the combination of the catalysis of Pd nanoparticles and the hollow structure. Based on the above reasons it is advisable that the Pd decorated hollow sphere SnO2 gas sensor is a promising candidate for good performance CO sensor. DFT calculation results also show that CO oxidation is easily and fast occurred on the Pd-doped SnO2 (110) surface with lower reaction barrier and more negative reaction energy. These calculation results will provide further understanding of CO oxidation process for gas-sensing, which is important to explain the experimental phenomenon.Acknowledgements
This work is supported by the National Nature Science Foundation of China (No. 61374218, 61134010, and 61327804, 61520106003) and Program for Chang Jiang Scholars and Innovative Research Team in University (No. IRT13018). National High-Tech Research and Development Program of China (863 Program, No. 2014AA06A505).Notes and references
- R. G. Pavelko, M. Yuasa, T. Kida, K. Shimanoe and N. Yamazoe, Sens. Actuators, B, 2015, 210, 719 CrossRef CAS.
- X. Li, C. Wang, X. Zhou, J. Liu, P. Sun and G. Lu, RSC Adv., 2014, 4, 47319 RSC.
- T. Hyodo, S.-i. Furuno, E. Fujii, K. Matsuo, S. Motokucho, K. Kojio and Y. Shimizu, Sens. Actuators, B, 2013, 187, 495 CrossRef CAS.
- J. S. Cho, Y. J. Hong, J. H. Lee and Y. C. Kang, Nanoscale, 2015, 7, 8361 RSC.
- J. W. Yoon, Y. J. Hong, G. D. Park, S. J. Hwang, F. Abdel-Hady, A. A. Wazzan, Y. C. Kang and J. H. Lee, ACS Appl. Mater. Interfaces, 2015, 7, 7717 CAS.
- M.-H. Seo, M. Yuasa, T. Kida, J.-S. Huh, N. Yamazoe and K. Shimanoe, Sens. Actuators, B, 2011, 154, 251 CrossRef CAS.
- J. S. Cho, J. M. Won, J. H. Lee and Y. C. Kang, Nanoscale, 2015, 7, 19620 RSC.
- C. Wang, X. Li, C. Feng, Y. Sun and G. Lu, Sens. Actuators, B, 2015, 210, 75 CrossRef CAS.
- A. Sanger, A. Kumar, A. Kumar and R. Chandra, Sens. Actuators, B, 2016, 234, 8 CrossRef CAS.
- A. Sanger, A. Kumar, A. Kumar, J. Jaiswal and R. Chandra, Sens. Actuators, B, 2016, 236, 16 CrossRef CAS.
- H. Wang and A. L. Rogach, Chem. Mater., 2014, 26, 123 CrossRef CAS.
- S. Basu and P. Bhattacharyya, Sens. Actuators, B, 2012, 173, 1 CrossRef CAS.
- J. Huang, L. Wang, C. Gu, Z. Wang, Y. Sun and J.-J. Shim, Sens. Actuators, B, 2015, 207, 782 CrossRef CAS.
- L. Wang, Z. Lou, J. Deng, R. Zhang and T. Zhang, ACS Appl. Mater. Interfaces, 2015, 7, 13098 CAS.
- S. Tian, X. Ding, D. Zeng, J. Wu, S. Zhang and C. Xie, RSC Adv., 2013, 3, 11823 RSC.
- W. S. Kim, B. S. Lee, D. H. Kim, H. C. Kim, W. R. Yu and S. H. Hong, Nanotechnology, 2010, 21, 245605 CrossRef PubMed.
- P. Sun, X. Zhou, C. Wang, K. Shimanoe, G. Lu and N. Yamazoe, J. Mater. Chem. A, 2014, 2, 1302 CAS.
- C. Wang, X. Cheng, X. Zhou, P. Sun, X. Hu, K. Shimanoe, G. Lu and N. Yamazoe, ACS Appl. Mater. Interfaces, 2014, 6, 12031 CAS.
- Y. Bing, Y. Zeng, S. Feng, L. Qiao, Y. Wang and W. Zheng, Sens. Actuators, B, 2016, 227, 362 CrossRef CAS.
- Y. Li, Y. Hu, H. Jiang, X. Hou and C. Li, RSC Adv., 2013, 3, 22373 RSC.
- M. Yuasa, T. Kida and K. Shimanoe, ACS Appl. Mater. Interfaces, 2012, 4, 4231 CAS.
- Y. Li, L. Qiao, D. Yan, L. Wang, Y. Zeng and H. Yang, J. Alloys Compd., 2014, 586, 399 CrossRef CAS.
- S. Vallejos, T. Stoycheva, F. E. Annanouch, E. Llobet, P. Umek, E. Figueras, C. Cane, I. Gracia and C. Blackman, RSC Adv., 2014, 4, 1489 RSC.
- D.-J. Yang, I. Kamienchick, D. Y. Youn, A. Rothschild and I.-D. Kim, Adv. Funct. Mater., 2010, 20, 4258 CrossRef CAS.
- A. A. Zhukova, M. N. Rumyantseva, V. B. Zaytsev, A. V. Zaytseva, A. M. Abakumov and A. M. Gaskov, J. Alloys Compd., 2013, 565, 6 CrossRef CAS.
- Y. C. Lee, H. Huang, O. K. Tan and M. S. Tse, Sens. Actuators, B, 2008, 132, 239 CrossRef CAS.
- X.-T. Yin and X.-M. Guo, Sens. Actuators, B, 2014, 200, 213 CrossRef CAS.
- D. Trung do, N. D. Hoa, P. V. Tong, N. V. Duy, T. D. Dao, H. V. Chung, T. Nagao and N. V. Hieu, J. Hazard. Mater., 2014, 265, 124 CrossRef PubMed.
- Q. Wang, X. Li, F. Liu, Y. Sun, C. Wang, X. Li, P. Sun, J. Lin and G. Lu, Sens. Actuators, B, 2016, 230, 17 CrossRef CAS.
- A. Kumar, A. Sanger, A. Kumar and R. Chandra, RSC Adv., 2016, 6, 47178 RSC.
- Q. Wang, C. Wang, H. Sun, P. Sun, Y. Wang, J. Lin and G. Lu, Sens. Actuators, B, 2016, 222, 257 CrossRef CAS.
- P. Bechthold, M. E. Pronsato and C. Pistonesi, Appl. Surf. Sci., 2015, 347, 291 CrossRef CAS.
- R. Cavicchi, M. Tarlov and S. Semancik, J. Vac. Sci. Technol., 1990, 8, 2347 CrossRef CAS.
- B. Delley, Phys. Rev. B, 2002, 66, 155 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B, 1976, 13, 5188 CrossRef.
- Materials Studio V4.0, San Diego, 2005 Search PubMed.
- Z. Lu, D. Ma, L. Yang, X. Wang, G. Xu and Z. Yang, Phys. Chem. Chem. Phys., 2014, 16, 12488 RSC.
- Y. Xue and Z. Tang, Sens. Actuators, B, 2009, 138, 108 CrossRef CAS.
- A. Robina, E. Germán, M. Pronsato, A. Juan, I. Matolínová and V. Matolín, Vacuum, 2014, 106, 86 CrossRef CAS.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |