Chitosan and imide-functional Fe3O4 nanoparticles to prepare new xanthene based poly(ether-imide) nanocomposites
Meisam Shabanian*a,
Hassan Moghanian b,
Mahroo Khaleghia,
Mohsen Hajibeygic,
Hossein Ali Khonakdard and
Henri Vahabi
b,
Mahroo Khaleghia,
Mohsen Hajibeygic,
Hossein Ali Khonakdard and
Henri Vahabi e
e
aFaculty of Chemistry and Petrochemical Engineering, Standard Research Institute (SRI), Karaj P. O. Box 31745-139, Iran. E-mail: m.shabanian@standard.ac.ir; Fax: +98-2632818844; Tel: +98-9129379872
bDepartment of Chemistry, Dezful Branch, Islamic Azad University, Dezful, Iran
cFaculty of Chemistry, Kharazmi University, 15719-14911 Tehran, Iran
dDepartment of Polymer Engineering, Faculty of Engineering, South Tehran Branch, Islamic Azad University, P. O. Box 19585-466, Tehran, Iran
eUniversité de Lorraine, LMOPS-EA 4423, Metz, F-57070, France
First published on 23rd November 2016
Abstract
In this work a new series of multifunctional nanocomposites were synthesized based on a soluble poly(ether-imide). The poly(ether-imide) was successfully synthesized via direct polycondensation reaction. A methyl rich diamine containing xanthene group as a new monomer was synthesized and used for polymerization. The xanthene based poly(ether-imide) was characterized using size exclusion chromatography (SEC) and nuclear magnetic resonance (NMR). Fe3O4 nanoparticles were imide-functionalized by a dianhydride, chitosan and phenylalanine. The functional Fe3O4 nanoparticles were incorporated into the new synthesized poly(ether-imide). Effects of the functionalized Fe3O4 nanoparticles on the thermal and combustion properties of the corresponding nanocomposites were investigated. Thermogravimetric analysis (TGA) results indicated that the thermal stability and char yields of the nanocomposites were enhanced compared to the neat poly(ether-imide). Microscale combustion calorimetry (MCC) revealed that the synthesized poly(ether-imide) had low flammability. The incorporation of functional Fe3O4 nanoparticles could further improve the combustion properties of poly(ether-imide). The high interaction between poly(ether-imide) and functional Fe3O4, the presence of an imide group and high hydroxyl content of the functional Fe3O4 nanoparticles seem to be responsible for the improvement of the thermal and combustion properties. Furthermore, the presence of methyl, ether and bulky xanthene groups in the polyimide backbone decreased the glass transition temperature (Tg) and increased the solubility in organic solvents. These properties will be useful for processing and new applications of poly(ether-imide).
1. Introduction
Aromatic polyimides (PI) are considered to be high-performance materials which make them useful for advanced technologies. PI could be an appropriate choice for the replacement of traditional materials including metals and ceramics in new technological applications.1,2 PI offer a wide range of properties including transparency, non-flammability, outstanding strength-to-weight ratios, thermal resistance, good barrier, and solvent resistant properties.3–7 However, poor solubility in common organic solvents and very high transition temperatures are the limiting factors for the processing and application of these materials. Recent basic and applied studies have focused on increasing their processability and solubility in order to enlarge the scope of the technological applications of these materials. It was known that the solubility of polymers often increased when flexible bonds such as [–O–, –SO2–, –CH2–], polar constituents and bulky pendent groups are incorporated into the polymer backbone by changing the intermolecular interactions and crystallinity.8–12Xanthenes are known as nature based compounds and are interesting for their utility as leuco-dyes,13 pH-sensitive fluorescent materials for the visualization of biomolecules14 and in laser technologies15 due to their outstanding spectroscopic properties. The introduction of xanthene group and its derivatives into polymer in order to improve the solubility and thermal stability has attracted several research efforts.16–18 It has been proved that the introduction of rigid heteroaromatic xanthene group as a bulky pendent group to the polymer backbone could endow the polymer with new properties such as photoluminescence, electroluminescence and liquid crystallinity. Xanthene group can also decrease the negative effects resulting from the introduction of flexible linkages such as aliphatic and ether groups in the aromatic polyimide backbone. Therefore, polymers containing xanthene groups are expected to have good solubility, easy processability, and excellent thermal properties.19–21
High-performance polymers, including PI, are ideal candidates for matrices in nanocomposite materials due to their unique properties such as high glass transition temperature (Tg), stiffness, strength, toughness, high chemical resistance, conductivity, thermo oxidative stability, outstanding flame resistance, low dielectric constant, stable properties at high temperatures, high thermal stability, long storage periods and recyclability.22,23 The preparation of polymer nanocomposite based on magnetic nanoparticles has attracted different applications. These nanocomposites represent a class of functional materials which may have some potential applications in batteries, electromagnetic interference shielding, electro magneto rheological fluids, electrochemical display devices and microwave absorbing materials.24–26 The main challenge in polymer nanocomposites is the prevention of nanoparticle aggregation. It was found that the preparation of well dispersed nanocomposites and the prevention of nanoparticle agglomeration in a polymer matrix were difficult due to their high specific surface area. However, researchers still have to face how to avoid the poor dispersions of Fe3O4 nanoparticles in polymeric matrix caused by the weak interfacial interactions between filler and polymer. Thus, developing polymer/Fe3O4 nanocomposites with excellent Fe3O4 dispersion in the polymer matrix has been an essential goal.27 The surface modification of the nanoparticles can improve the interactions between the nanoparticles and the polymer matrix.28,29
In this paper, we deal with a new poly(ether-imide) nanocomposite that is a soluble multifunctional polyimide (PIX: polyimide xanthene) reinforced with chitosan–imide-functional Fe3O4 nanoparticles ((Fe3O4)SiO2@Ch–Im: chitosan–imide-functional magnetic nanoparticles). The new polyimide with ether, methyl and bulky xanthene groups could have high potential application due to multifunctionality and ease of processability. However, chitosan–imide-functional Fe3O4 nanoparticles as a new reinforcement are a potential candidate to improve properties and endow some new applications. The thermal stability and combustion behaviour of the PIX/functional Fe3O4 nanocomposite were studied by TGA and microscale combustion calorimeter (MCC).
2. Experimental
2.1 Materials
2,5-Dimethylphenol (2,5-xylenol), terephthalaldehyde, 4-toluene sulfonic acid monohydrate (p-TSA), pyromellitic anhydride, 1-fluoro-4-nitrobenzene, 2-naphthol 3,3′,4,4′-benzophenonetetracarboxylic dianhydride (BPD), [3-(2,3-epoxypropoxy)propyl]trimethoxysilane (EPO) and chitosan were purchased from Sigma-Aldrich. 2-Naphthol, FeCl2·4H2O, FeCl3·6H2O, palladium charcoal (Pd/C), N,N-dimethylformamide (DMF) and hydrazine hydrate from Merck were used without further purification.2.2 Synthesis of 4-(4-((4-(14H-dibenzo[a,j]xanthen-14-yl)phenyl)(2,6-dimethyl-4-(p-tolyloxy)phenyl)methyl)-3,5-dimethylphenoxy)aniline
The diamine containing xanthene, methyl and ether groups was synthesized starting from 2-naphthol and terephthalaldehyde in four-step reactions according to the following procedure (Scheme 1).![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added. Yield = 90%; melting point (Mp): 330–332 °C.
1) was added. Yield = 90%; melting point (Mp): 330–332 °C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was added. The suspension was stirred for 10 min and then the precipitate was filtered. Yield = 86%; Mp: 310–312 °C.
1) was added. The suspension was stirred for 10 min and then the precipitate was filtered. Yield = 86%; Mp: 310–312 °C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was prepared in a 250 mL round-bottomed flask. The mixture was heated and hydrazine monohydrate 80% (10 mL) in ethanol (15 mL) was added dropwise during 30 min through a dropping funnel. The mixture was heated to reflux for 2 h at 70 °C and then was filtered while hot to remove Pd/C. The solvent was evaporated under vacuum and the product was washed thoroughly with ethanol, and dried to recover the white solid product (Scheme 1). Yield = 91%; Mp = 182–184 °C.
1) was prepared in a 250 mL round-bottomed flask. The mixture was heated and hydrazine monohydrate 80% (10 mL) in ethanol (15 mL) was added dropwise during 30 min through a dropping funnel. The mixture was heated to reflux for 2 h at 70 °C and then was filtered while hot to remove Pd/C. The solvent was evaporated under vacuum and the product was washed thoroughly with ethanol, and dried to recover the white solid product (Scheme 1). Yield = 91%; Mp = 182–184 °C.2.3 Synthesis of PIX
To synthesize poly(amic acid) (PAA), 6.36 g (8 mmol) of BXNH2 was put into a three-necked flask containing 25 mL of DMF at room temperature. BXNH2 was completely dissolved, and then a solution of 1.74 g (8 mmol) of pyromellitic dianhydride in 10 mL of DMF was slowly added to the BXNH2 solution. The solution was stirred at room temperature overnight to prepare PAA solution. Then the PAA solution was poured into a glass dish and heated with a programmed temperature at 70, 100, 150 and 200 °C for 8 h in a vacuum oven.2.4 Synthesis of chitosan and imide-functional Fe3O4 nanoparticles (Fe3O4)SiO2@Ch–Im
Fe3O4 nanoparticles were synthesized according to the literature.30 Briefly, FeCl2·4H2O (2.39 g, 12 mmol) and FeCl3·6H2O (6.49, 24 mmol) were dissolved in deionized water (200 mL) under N2 atmosphere with vigorous stirring using a mechanical stirrer. 25 mL of NH3·H2O (28 wt%) was added to the solution for 30 min and then was heated to 60 °C for 6 h. Then, the temperature was increased to 85 °C to vaporize residual ammonia. The nanoparticles were separated by a magnet and washed with deionized water.2.5 Preparation of poly(ether-imide)/functional Fe3O4 nanocomposites (PIXN)
The poly(ether-imide)/functional Fe3O4 nanocomposites were prepared using the solvent-casting method. To prepare PIXN with 1 and 3 wt% of (Fe3O4)SiO2@Ch–Im loading (PIXN 1 and PIXN 3), PIX was dissolved in DMF at 120 °C in a three-necked flask with mechanical stirring and ultrasonic irradiation to form a homogeneous solution. Then, (Fe3O4)SiO2@Ch–Im was dispersed in DMF and added into the PIX solution. The mixture was stirred and sonicated subsequently for 1 day. The blended mixture was cast onto glass plates and dried at 40 °C in an oven for 24 h to form the nanocomposites (Scheme 3).2.6 Characterization
Fourier transform infrared (FTIR) spectra were obtained using a Perkin-Elmer RXI spectrometer in the range of 400–4000 cm−1 with a resolution of 2 cm−1. 1H NMR measurements were performed using a Bruker 300 MHz spectrometer. Molar mass (mass-average (Mw) and number-average (Mn) molar mass) were determined thanks to size exclusion chromatography (SEC) using Agilent Series 1100 (Agilent) system equipped with a pump, degasser and differential refractive index (RI) detector. UV-Vis spectra were recorded at 25 °C in the 190–790 nm spectral regions with a Perkin Elmer Lambda 15 spectrophotometer in DMF using cell lengths of 1 cm. The morphology of the nanocomposites was studied using transmission electron microscopy (TEM) with microscope LEO 912. Thermogravimetric analysis (TGA) was undertaken using a TA Instruments TGAQ5000 in the range between room temperature and 800 °C at a heating rate of 10 °C min−1 in nitrogen atmospheres. Differential scanning calorimetry (DSC) (TA instrument Q1000) was used to study the thermal degradation properties. Three cycles of heating–cooling–heating with heating/cooling rate of 10 °C min−1 were used. Combustion properties of the samples were studied by microscale combustion calorimeter (MCC, FTT) and repeated three times for each sample.3. Results and discussions
3.1 Synthesis of the diamine
The new methyl rich diamine containing xanthene ring and ether group was prepared starting from 2-naphthol and terephthalaldehyde via a four-step reaction sequence, as outlined in Scheme 1. An equivalent of terephthalaldehyde with two equivalents of 2-naphthol in the presence of p-TSA as a catalyst were used to synthesize BX via one-pot condensation reaction. Then, the new methyl rich bisphenol containing xanthene ring (BXOH) was synthesized by a chemical synthesis pathway from 2,5-dimethylphenol and BX in the presence of p-TSA under solvent-free conditions. After recrystallization of BXOH in a mixture of ethanol–water, it was used in the next step of the reaction. In a mixture of DMF and K2CO3, BXOH was reacted with 1-fluoro-4-nitrobenzene to obtain BXNO2. Hydrazine monohydrate and palladium on charcoal (10%) was used for reduction of BXNO2 to BXNH2 as a novel multifunctional diamine.The 1H NMR spectrum of BXNH2 displays the characteristic resonance of methyl groups at 1.86 and 1.87, NH2 amine groups at 4.87 ppm and C–H center at 5.31 ppm. The aromatic protons appeared in rang between 6.23 and 8.68 ppm (Fig. 1).
The 13C NMR spectrum of BXNH2 in dimethyl sulfoxide (DMSO-d6) is presented in Fig. 2. The four signals of aliphatic carbons appear in the region of 16–47 ppm. The carbon of the aromatic rings appears in the downfield (114–154 ppm) which confirms its chemical structure.
3.2 Polymer synthesis and characterization
In line with our previous works to introduce flexible, aliphatic and side chains group with different functionality for improving the processability and decreasing Tg of aromatic polyamides and polyimides without loss of their important properties such as thermal stability, in this work, the new poly(ether-imide) containing xanthene ring along with ether groups and high methyl content was synthesized and characterized. Xanthene rings in the side chain of polymer could be a successful approach to improve the solubility of the polymers.Xanthene, ether and aliphatic groups in the PIX chains improved solubility of the resulting poly(ether-imide). The polymer was dissolved in polar organic solvents such as DMSO, DMF at room temperature and was insoluble in protic solvents such as methanol, ethanol and water.
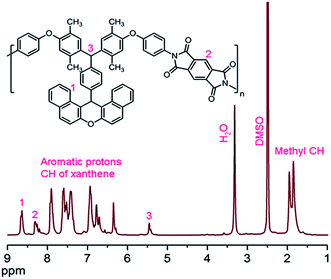
The chemical structure of newly synthesized PIX was confirmed by means of 1H-NMR spectrum (Fig. 3). In the 1H-NMR spectrum the resonance of methyl protons appeared in the range of 1.82–1.93 ppm. The aromatic protons and an aliphatic proton related to CH xanthene appeared in the range of 6.70–8.81 ppm. The presence of resonance signals related to BXNH2 and pyromellitic dianhydride in 1H NMR spectrum confirmed the chemical structure of PIX.
The SEC measurement of the synthesized PIX exhibited number-average molar mass (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) n) and mass-average molar mass (
n) and mass-average molar mass (![[M with combining macron]](https://www.rsc.org/images/entities/i_char_004d_0304.gif) w) of ca. 10
w) of ca. 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and 25
100 and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g mol−1, respectively, according to poly(vinylpyridine) (PVP) standard. The polydispersity index was 2.5.
200 g mol−1, respectively, according to poly(vinylpyridine) (PVP) standard. The polydispersity index was 2.5.
3.3 Synthesis of (Fe3O4)SiO2@Ch–Im
The multifunctional Fe3O4 nanoparticles ((Fe3O4)SiO2@Ch–Im) were synthesized via four steps (Scheme 2). The FTIR spectra of the (Fe3O4)SiO2 and (Fe3O4)SiO2@Ch–Im are shown in Fig. 4. The chitosan and imide coated onto (Fe3O4)SiO2 by coupling agent EPO can be clearly observed thanks to the strong absorption bands at 1776 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O antisymmetric, imide), 1713 cm−1 (C
O antisymmetric, imide), 1713 cm−1 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O symmetric, imide) and the bands at around 2931 and 2860 cm−1 which are attributed to the aliphatic C–H bonds in chitosan, phenylalanine and EPO. The absorption band at 1386 cm−1 (C–N, imide) showed the presence of the imide heterocycle in the Fe3O4 nanoparticle. The absorption bands at 1100 and 558 cm−1 correspond to the Si–O–Si and Fe–O bonds. The strong OH band (3413 cm−1) assigned to the stretching vibrations of Fe–OH groups absorbed on the surface of (Fe3O4)SiO2 and the OH of chitosan. These results revealed that the macromolecular chains of chitosan and imide are chemically linked on the surfaces of (Fe3O4)SiO2 in a coupling manner, and that (Fe3O4)SiO2@Ch–Im was formed.
O symmetric, imide) and the bands at around 2931 and 2860 cm−1 which are attributed to the aliphatic C–H bonds in chitosan, phenylalanine and EPO. The absorption band at 1386 cm−1 (C–N, imide) showed the presence of the imide heterocycle in the Fe3O4 nanoparticle. The absorption bands at 1100 and 558 cm−1 correspond to the Si–O–Si and Fe–O bonds. The strong OH band (3413 cm−1) assigned to the stretching vibrations of Fe–OH groups absorbed on the surface of (Fe3O4)SiO2 and the OH of chitosan. These results revealed that the macromolecular chains of chitosan and imide are chemically linked on the surfaces of (Fe3O4)SiO2 in a coupling manner, and that (Fe3O4)SiO2@Ch–Im was formed.
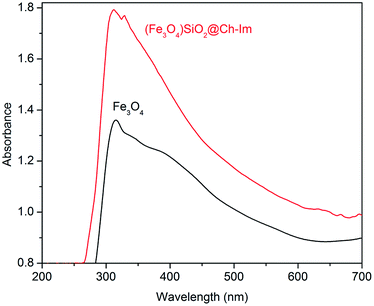
UV-Vis was used to evaluate the formation of Fe3O4 and (Fe3O4)SiO2@Ch–Im nanoparticles in suspension. As shown in Fig. 5, observed absorbance peaks at around 315, 358 and 400 nm can be attributed to [Fe2+]t2g → [Fe2+]e, [Fe3+]eg → [Fe2+]t2 and [Fe2+]t2g → [Fe2+]t2 transitions of Fe3O4 nanoparticles respectively, according to the W. F. J. Fontijn et al. assignments.32 (Fe3O4)SiO2@Ch–Im nanoparticle showed an obvious absorption peak appeared at 329 nm, which can be attributed to π–π* transitions of the benzenoid rings, indicating the successful functionalization on the surface of Fe3O4 nanoparticles. Furthermore, the weak bound at around 639 nm correspond to the [Fe2+]t2g → [Fe2+]eg transition.
3.4 Properties of the nanocomposites
| Samples | T5a (°C) | T10a (°C) | Char yieldb | Tgc |
|---|---|---|---|---|
| a Temperature at which 5% and 10% weight loss was recorded by TGA at a heating rate of 10 °C min−1.b Weight percentage residue of the material after TGA analysis at a maximum temperature of 800 °C.c Glass transition temperature was recorded at a heating rate of 10 °C min−1 in a nitrogen atmosphere. | ||||
| PIX | 430 | 454 | 47 | 178 |
| PIXN 1 | 438 | 456 | 50 | 180 |
| PIXN 3 | 439 | 456 | 51 | 188 |
The glass transition temperatures values of the neat PIX and the nanocomposites were investigated by DSC. The incorporation of xanthene side group, ether and methyl units into the PIX back-bone resulted an aromatic polyimide with low Tg of 178 °C as compared to conventional aromatic polyimide. A slight increase in Tg for PIXN 1 and PIXN 3 was observed. Dispersion and interaction of nanoparticles with polymer matrix controlled Tg. This increase could be explained by high interaction between (Fe3O4)SiO2@Ch–Im and the PIX matrix which restricts the chains movements.
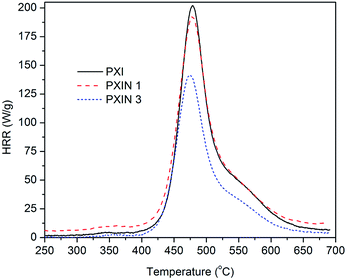
The HRR curves of PIX and its nanocomposites are presented in Fig. 8 and the corresponding combustion data are reported in Table 2. The peak heat release rate (pHRR) value of PIX was 203 W g−1. It was observed that the pHRR value decreases to 197 W g−1, with loading 1 wt% (Fe3O4)SiO2@Ch–Im, indicating an improvement in the combustion data of PIX. The pHRR value of PIXN 3 was 141 W g−1, which showed a further improvement as compared with PIXN 1. HRC is also an important parameter used to predict and evaluate flame behaviour. Table 2 shows HRC value of 267 J g−1 K−1 for PIX, while the (Fe3O4)SiO2@Ch–Im nanocomposites show lower HRC values. Total heat release (THR) calculated from the area under the HRR curve is another important parameter. THR value of PIX was 14 kJ g−1 and this parameter reached 13.3 kJ g−1 in PIXN 1. By increasing the (Fe3O4)SiO2@Ch–Im content, the THR value decreased to 10 kJ g−1. As compared to the neat PIX, 26% improvements in the THR value was obtained with an increase in the (Fe3O4)SiO2@Ch–Im loading to 3 wt%. It can be concluded that the most efficient results were achieved with loading of 3 wt% of (Fe3O4)SiO2@Ch–Im in the PIX matrix. It is well known that the condensed phase actions could not be detected in MCC because of small amounts of materials. Therefore, it is possible that PIXN 3 sample represents the better flame retardancy properties in cone calorimeter test, in addition to its action in gas phase which has been detected in MCC.
| Samples | pHRR (W g−1) | HRC (J g−1 K−1) | THR (kJ g−1) | Tmax |
|---|---|---|---|---|
| PIX | 203 | 267 | 14 | 478 |
| PIXN 1 | 192 | 187 | 13 | 489 |
| PIXN 3 | 141 | 143 | 10 | 485 |
4. Conclusion
In this investigation, the new poly(ether-imide) bearing a xanthene ring pendent group along with ether and methyl groups has been synthesized. Thanks to the incorporation of the functionalities, flexibility and solubility of PIX were improved. It also increased the inter-chain spacing or free volume, thus decreased glass transition temperature. A new functional Fe3O4 with chitosan and imide groups was synthesized and incorporated into the poly(ether-imide) matrix. The functionalized Fe3O4 have been successfully prepared by surface modification with chitosan, the dianhydride, phenylalanine and EPO. The introduction of these groups increased interaction between the nanoparticle and the poly(ether-imide) matrix. (Fe3O4)SiO2@Ch–Im could form strong hydrogen bonds with the PIX matrix and the results revealed showed good properties enhancement. TGA results showed that PIX has high thermal stability and char residue and incorporation of (Fe3O4)SiO2@Ch–Im improved the thermal properties of PIX. From MCC data, it has been found that (Fe3O4)SiO2@Ch–Im has a positive effect on improving the combustion data of PIX, the decrease in peak heat release rate (pHRR) and the total heat release (THR) of the PIXN nanocomposites. It was concluded that the best results, thermal and flammability, were obtained when 3 wt% of (Fe3O4)SiO2@Ch–Im was incorporated to the PIX matrix. Thus these nanocomposites classified as a new processable high performance materials.Acknowledgements
This work was financially supported by grants from INSF (Iran National Science Foundation) (Grant No. 930823).References
- N. Asano, M. Aoki, S. Suzuki, K. Miyatake, H. Uchida and M. Watanabe, J. Am. Chem. Soc., 2006, 128, 1762–1769 CrossRef CAS PubMed.
- A. Tena, Á. Marcos-Fernández, M. de la Viuda, L. Palacio, P. Prádanos, Á. E. Lozano, J. de Abajo and A. Hernández, Eur. Polym. J., 2015, 62, 130–138 CrossRef CAS.
- Y. M. Xu, N. L. Le, J. Zuo and T.-S. Chung, J. Membr. Sci., 2016, 499, 317–325 CrossRef CAS.
- T. Namikoshi, K. Odahara, A. Wakino, M. Murata and S. Watanabe, High Perform. Polym., 2015, 27, 183–190 CrossRef CAS.
- M. Shabanian, H. Ardeshir, S. Haji-Ali, H. Moghanian, M. Hajibeygi, K. Faghihi, H. A. Khonakdar and H. Salimi, Appl. Clay Sci., 2016, 285–291 CrossRef CAS.
- M. Shabanian, K. Faghihi, A. Raeisi, M. Varvanifarahani, H. A. Khonakdar and U. Wagenknecht, J. Therm. Anal. Calorim., 2014, 117, 293–299 CrossRef CAS.
- D.-J. Liaw, K.-L. Wang, Y.-C. Huang, K.-R. Lee, J.-Y. Lai and C.-S. Ha, Prog. Polym. Sci., 2012, 37, 907–974 CrossRef CAS.
- M.-H. Tsai, I.-H. Tseng, S.-L. Huang and C.-W. Hsieh, Int. J. Polym. Mater. Polym. Biomater., 2014, 63, 48–56 CrossRef CAS.
- W.-H. Liao, S.-Y. Yang, S.-T. Hsiao, Y.-S. Wang, S.-M. Li, H.-W. Tien, C.-C. M. Ma and S.-J. Zeng, RSC Adv., 2014, 4, 51117–51125 RSC.
- S. Xia, Z. Sun, L. Yi and Y. Wang, RSC Adv., 2013, 3, 14661–14670 RSC.
- L. Yi, C. Li, W. Huang and D. Yan, Polymer, 2015, 80, 67–75 CrossRef CAS.
- F. Atabaki and H. Ahmadizadegan, Polym.–Plast. Technol. Eng., 2015, 54, 523–531 CrossRef CAS.
- H. Moghanian, A. Mobinikhaledi and Z. Baharangiz, J. Polym. Res., 2014, 21, 1–16 CAS.
- R. C. Hunter and T. J. Beveridge, Appl. Environ. Microbiol., 2005, 71, 2501–2510 CrossRef CAS PubMed.
- T. Li, S. R. Sheng, M. H. Wei, C. Chen and C. S. Song, Chin. Chem. Lett., 2010, 21, 1247–1250 CrossRef CAS.
- M. Hajibeygi, M. Shabanian, H. Moghanian, H. Khonakdar and L. Häußler, RSC Adv., 2015, 5, 53726–53735 RSC.
- C. D. Simone and T. E. Carney, US Pat., US8969909 B2, 2015.
- P. Bandyopadhyay and S. Banerjee, Eur. Polym. J., 2015, 69, 140–155 CrossRef CAS.
- D.-D. Guo, S.-R. Sheng, X.-Y. Sang, Z.-Z. Huang and X.-L. Liu, J. Macromol. Sci., Part A: Pure Appl. Chem., 2015, 52, 950–959 CrossRef CAS.
- H. Moghanian, A. Mobinikhaledi and R. Monjezi, Des. Monomers Polym., 2015, 18, 157–169 CrossRef CAS.
- D. D. Guo, S. R. Sheng, X. C. Mao, X. L. Liu and J. W. Jiang, High Perform. Polym., 2016, 28, 309–314 CrossRef CAS.
- T. J. Fai, J. E. Mark and P. N. Prasad, Polymers and Other Advanced Materials Emerging Technologies and Business Opportunities, Springer, 1996 Search PubMed.
- A. M. Díez-Pascual, M. Naffakh, C. Marco, G. Ellis and M. A. Gómez-Fatou, Prog. Mater. Sci., 2012, 57, 1106–1190 CrossRef.
- R. T. Araujo, G. R. Ferreira, T. Segura, F. G. Souza and F. Machado, Eur. Polym. J., 2015, 68, 441–459 CrossRef CAS.
- S. W. Song, Y. Jeong and S. Kwon, IEEE J. Sel. Top. Quantum Electron., 2015, 21, 1–12 CrossRef CAS.
- J. Wilson, P. Poddar, N. Frey, H. Srikanth, K. Mohomed, J. Harmon, S. Kotha and J. Wachsmuth, J. Appl. Phys., 2004, 95, 1439–1443 CrossRef CAS.
- S. Kango, S. Kalia, A. Celli, J. Njuguna, Y. Habibi and R. Kumar, Prog. Polym. Sci., 2013, 38, 1232–1261 CrossRef CAS.
- I.-Y. Jeon and J.-B. Baek, Materials, 2010, 3, 3654–3674 CrossRef CAS.
- M. Shabanian, N.-J. Kang, D.-Y. Wang, U. Wagenknecht and G. Heinrich, RSC Adv., 2013, 3, 20738–20745 RSC.
- B. Mu, P. Liu, Y. Dong, C. Lu and X. Wu, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 3135–3144 CrossRef CAS.
- A. Chrobok, S. Baj, W. Pudło and A. Jarzębski, Appl. Catal., A, 2009, 366, 22–28 CrossRef CAS.
- W. Fontijn, P. Van der Zaag, M. Devillers, V. Brabers and R. Metselaar, Phys. Rev. B: Condens. Matter Mater. Phys., 1997, 56, 5432 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |