Synthetic methods and electrochemical applications for transition metal phosphide nanomaterials
Yao Lu
a,
Tianyi Wang
a,
Xinran Li
ab,
Guangxun Zhang
a,
Huaiguo Xue
*a and
Huan Pang
 *ab
*ab
aCollege of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, 225002, Jiangsu, China. E-mail: huanpangchem@hotmail.com; panghuan@yzu.edu.cn; chhgxue@yzu.edu.cn
bCollege of Chemistry and Chemical Engineering, Anyang Normal University, Anyang, 455002, Henan, China
First published on 16th August 2016
Abstract
Currently, the great demand for energy has triggered the exploration of new devices for energy storage and conversion. For these devices, electrode materials with excellent performance are strongly needed. In particular, transition metal phosphide nanomaterials are emerging with excellent performances. Transition metal phosphide nanomaterials have been utilized in Li-ion batteries, Na-ion batteries, supercapacitors, solar cells, electrocatalysis, etc. This review summarizes recent developments and challenges in transition metal phosphide nanomaterials, with a focus on synthetic methods and electrochemical applications.
1. Introduction
The development of modern society largely depends on natural resources, such as oil and coal. However, at the same time, a number of problems are emerging due to the overconsumption of these resources,1 like energy shortages, air pollution and global warming.2–5 Thus, the demand for energy is urgent and so is the development of renewable energy sources.6–12 Therefore, clean energy has received great attention as a promising substitute in the past few years. Two successful examples are wind turbines and solar cells.The utilization of electrical energy can be also perceived as a special symbol of the second industrial revolution and human society stepped into the age of electricity from that time on. The great improvements in our living standards and social production depend on electrical energy to a large extent today in the 21st century. As a consequence, the requirement for high-efficiency electrical energy storage and conversion is mandatory and urgent.13 Batteries and supercapacitors are excellent methods for electrochemical energy storage, while solar cells can be used for energy conversion.
Recent research regarding sustainable energy sources, as well as energy storage and conversion methods, concentrates on the exploitation of excellent materials in the fields of electrochemical energy storage and conversion. In the past, large quantities of research based on new materials focused on oxygenated chemicals,14–22 nitrogenous compounds23–25 and sulfocompounds,26 and so forth, whereas research aimed at phosphorous compounds was rare correspondingly. In recent years, phosphorous compounds have been developed and have turned into hot spots in the field of electrochemistry. The element P belongs to the nitrogen group but holds multi-electron orbitals, so P is likely to possess superior chemical properties compared to the N atom. Due to native properties in its structure, P shows excellent performances in electrochemical applications.27 Referring to recent research, a great variety of phosphorous compounds can be considered as nice choices in the electrochemistry field. Nevertheless, limited by space, we merely discuss transition metal phosphides, and other compounds containing phosphorus will be omitted.28
The use of nanomaterials is regarded as a kind of excellent strategy to enhance physical properties and chemical performances.29–31 When used in transition metal phosphides, nanomaterials can similarly display excellent properties.32 Thus, studies of transition metal phosphides are mainly based on nanomaterials. Herein, we deliver a brief account of synthesis strategies for transition metal phosphide nanomaterials (TMPNs) firstly, and ball milling methods, electrodeposition synthetic methods, phosphorization, solution-phase synthetic methods, solid-phase synthetic methods and many other synthetic methods are introduced. Secondly, applications, for instance Li-ion batteries (LIBs), Na-ion batteries (NIBs), supercapacitors (SCs), solar cells and electrocatalysis, are discussed. It should be noted that there are quantities of research on TMPN catalysis that have been performed before, especially the research of Sun et al.10,11,33–41 Furthermore, Wang et al. summarized recent advances regarding cobalt-based heterogeneous electrocatalysts in water splitting in detail,42 and we do not elaborate on applications of TMPNs in the catalytic/electro-catalytic fields. Eventually, summaries and the outlook for future research are put forward.
2. Synthetic methods
Generally, TMPNs can be synthesized through solution-phase reactions,43–46 solid-phase reactions,27,47–51 and so forth.52 However, when TMPNs are used in the field of electrochemistry, some of the synthetic strategies are different from those used in other fields. In this case, we summarize several novel methods propitious to electrochemistry as well as TMPNs, for instance ball milling methods, electrodeposition methods, phosphorization, and many other synthetic methods. These methods are recommended according to specific circumstances.2.1 Ball milling methods
Lots of research based on such methods has been performed, while the ball milling method has been adopted in much research which is not involved with TMPNs, such as for a nanostructured Fe–8P (wt%) powder mixture.53 And Brutti et al. investigated the redox activity of MgH2 by assessing what effect would be caused as a result of ball milling pre-treatment.54In this review, we introduce ball milling synthetic methods for TMPNs. The ball milling method is a mechanochemical method utilizing the impact caused by the grinding medium (such as steel balls, pebbles, etc.) and the grinding effect of the grinding body and ball mill lining, and in this case, the effect of materials mixing and crushing can be reached. Several instances have been taken to illustrate its application in the synthesis of TMPNs and, tersely, we introduce these detailed applications, according to our discussion. Li et al. successfully synthesized pure GeP5 and GeP5/C at ambient temperature and pressure by utilizing a ball milling method.55 And a Cu3P sample was synthesized by Stan et al. via a ball milling method with n-dodecane existing.56 In addition, Kim et al. prepared tin phosphide (Sn4P3) through ball milling.57 A Sn4+xP3@(Sn–P) composite was prepared by Li et al. on a large scale, yet with low speed, through ball milling between P and Sn powders.58 Li et al. synthesized CoP nanoparticles using a productive, gentle and simple ball-milling strategy.59
2.2 Electrodeposition synthetic methods
Electrochemical deposition, or electrodeposition for short, is a method utilizing passing currents from external electrochemical batteries. Electrodeposition is a fascinating phenomenon. To the contrary, electroless deposition, where no external current flows, predates it by centuries. Since electricity was found and human beings put electricity into use with electrodeposition, electrodeposition has been exceptionally versatile, and valuable applications keep being invented. The electrodeposition process should be conducted under certain electrolytes as well as experimental conditions, and furthermore, the complexity of electrodeposition and the morphology of the sediment is connected with the properties of electrodeposition, relying also on the composition and pH of the electrolyte, as well as other factors.In this review, we give a brief overview with respect to the synthesis of TMPNs. To give an example, Chandrasekar et al. prepared iron phosphide over a copper substrate through the electrodeposition technique by utilizing an aqueous acidic electrolyte.60 Much other research concerning electrodeposition has also mushroomed. Nickel phosphide films were prepared by Lu et al. via the electrodeposition of four kinds of Ni films using choline chloride–ethylene glycol.61 Using electrodeposition, Xiang et al. fabricated porous nickel phosphide films using polystyrene sphere multi-layers as the template.62
2.3 Phosphorization
Phosphorization is also perceived as a general and effective strategy. Uniform yolk–shell Sn4P3@C nanospheres could be successfully synthesized through a phosphatization process with yolk–shell Sn@C nanospheres.63 Also, hierarchical nanostructured nickel phosphide (h-Ni2P) spheres can be prepared through a one-pot reaction in which nickel acetylacetonate is regarded as a precursor and trioctylphosphine (TOP) is taken as a phosphorus source, and the surfactant could be tri-n-octylamine as well as oleylamine (OAm).64 Bai et al. smoothly synthesized a Ni2P/C composite, which is like peapods, using NiNH4PO4H2O nanorods as templates.65 Hall et al. obtained amorphous FeP2 through the reaction of Fe(N(SiMe3)2)3 with PH3 in THF at 100 °C.662.4 Solution-phase synthetic methods
Solution-phase reactions have been adopted in the synthesis of TMPNs. And much research has been done since now. For instance, Ni2P/SiO2 catalysts can be synthesized using a solution-phase reaction in the presence of an SiO2 support, using Ni(acac)2 and trioctylphosphine.67 In addition, the novel solution-phase low temperature synthesis of a supported Ni2P catalyst has been described. And this used nickel acetylacetonate (Ni(acac)2) and low cost triphenylphosphine (TPP) in the presence of a coordinating solvent tri-n-octylamine (TOA) to form Ni2P/MCM-41 catalysts.45 Chen et al. reported the synthesis of single-crystalline Ni2P nanowires. And they used a solution-phase approach, using nickel(II) acetylacetonate as a metal precursor and trioctylphosphine as a phosphorus source.68 Li et al. successfully synthesized monodispersed nickel phosphide (Ni2P) nanorods and nanoparticles via a one-step solution-phase route, in which a mixture of trioctylphosphine oxide (TOPO) and trioctylphosphine (TOP) was used as the solvent, capping agent and phosphor source.692.5 Solid-phase synthesis methods
TMNPs can also be synthesized through solid-phase reactions. Ni2P nanoparticles were simply prepared through a solid phase reaction between H2PO2− and Ni2+ in N2 at relatively low temperatures. And these exhibited high activity for the hydrodesulfurization of dibenzothiophene and the hydrodenitrogenation of quinoline.27 What is more, Pfeiffer et al. prepared copper phosphide (Cu3P) thick films over copper foils via a solid-state reaction at low temperature (400 °C).50 A solid phase reaction between nickel cations and hypophosphites might occur at 473 K in the pores of mesoporous carbon (MC), resulting in highly dispersed Ni2P on the surface of the MC support. The Ni2P/MC catalysts thus prepared exhibited higher activities for the hydrodesulfurization (HDS) of dibenzothiophene (DBT) and the hydrodenitrogenation (HDN) of quinoline than Ni2P/SiO2 catalysts prepared through the reduction of phosphate at high temperatures.492.6 Other synthetic methods
There exist a large number of other strategies to synthesize TMPNs applied in the field of electrochemistry as well. Hydrothermal reactions,70,71 solvothermal synthetic methods,72,73 thermal decompositions, low-temperature solid state reaction methods, and thin-film vapor–liquid–solid (TF-VLS) synthetic strategies are all efficient and undemanding methods. At 180 °C, the hydrothermal reaction of red phosphorus, nickel chloride and graphene oxide results in nickel phosphide-embedded graphene, which is in an aqueous solution of ethylene glycol.74 Via a solvothermal reaction at 180 °C for 10 h, tin phosphide (Sn4P3) particles can be synthesized with different particle sizes.75 Similarly, nickel phosphide (NiP) nanoparticles could be prepared using a facile one-pot solvothermal method.76As for thermal decomposition, Keigo et al. successfully synthesized nickel phosphide through thermal decomposition, where nickel is regarded as a precursor and trioctylphosphine and trioctylphosphine oxide can be used as phosphorus sources.77 Furthermore, thermal decomposition can be utilized to synthesize single-crystalline Ni2P nanowires that possess a uniform diameter of about 8 nm and a length of about 100–200 nm, using syringe pumps to continuously deliver Ni–TOP (trioctylphosphine) complexes.78 Besides the methods above, low-temperature solid-state reactions are an excellent and general strategy. By utilizing this method, An et al. successfully synthesized Ni2P nanoparticles, which were synthesized on reduced graphene oxide (abbreviated as rGO).79 Furthermore, Zheng et al. investigated solar cells, which were based on InP, that were synthesized via non-epitaxial thin-film vapor–liquid–solid (TF-VLS) growth strategies.80
3. Electrochemical energy storage
3.1 Lithium storage – Li-ion batteries
Li-ion batteries (LIBs) can be defined as a means of satisfying our need for energy storage, which is key to exploiting alternative energy.81,82 And due to their superior performance, including high voltage, high capacity, high specific energy (in other words, high energy density), excellent power density, no memory effects, long-term cycle lifetimes, low self-discharge properties and environmental benignity in practical use,83–91 LIBs have drawn a great deal of attention. More importantly, LIBs have been widely used in portable electronic devices, and they can potentially possess wider application prospects when utilized as power sources in hybrid electric vehicles (HEVs) as well as electric vehicles (EVs).81,92–103Quantities of research have been performed to promote alternative electrode materials, which have specific energy densities suitable for LIBs and a higher Li+ utilization, in that the electrode materials determine the energy density of the batteries in significant measure. Currently, several electrode materials from TMPNs are designated as promising materials for LIBs, in addition, TMPNs with superior performance could be used as both anode materials and negative electrode materials. Some of the following mentioned materials are listed in Table 1.
| Electrode | Specific capacity/mA h g−1 | Rate | Cycles | Ref. |
|---|---|---|---|---|
| NiP2 | 750 | 0.13 mA cm−2 | 10 | 77 |
| Ni3P | 557 | 0.2C | 50 | 62 |
| Ni3P–Ni | 360 | 0.02 mA cm−2 | 100 | 104 |
| Ni2P | 398.5 | 54.2 mA g−1 | 50 | 61 |
| Ni12P5 | 600 | — | 200 | 105 |
| Ni5P4/C | 644.1 | 0.1C | 50 | 48 |
| Ni2P/C | 628 | 100 mA g−1 | 200 | 65 |
| Ni2P | 379.8 | 0.1C | 50 | 106 |
| Ni2P | 434 | 0.1C | 50 | 78 |
| Ni2P | 365.3 | 0.5C | 50 | 64 |
| FeP2 | 906 | 0.1C | 10 | 66 |
| GeP5/C | 2300 | 5 A g−1 | 40 | 55 |
| Sn4P3 | 315 | 200 mA g−1 | 200 | 75 |
| CoP | 839.1 | 5 μA cm−2 | 25 | 107 |
The crystal phases as well as the morphologies of the as-prepared materials were characterized utilizing XRD, as well as TEM. Utilizing nickel acetate tetrahydrate in trioctylphosphine oxide, NiP2 particles possessing 200–500 nm diameters were acquired at 360 °C for 5 h, while spherical Ni5P4 particles possessing 500 nm diameters were obtained with a synthetic process with a time of 1 h, with other conditions similar to those for NiP2. All-solid-state cells composed of NiP2 particles and 80Li2S center dot 20P2S5 (mol%) glass ceramic displayed excellent performance, with a discharge capacity of 1100 mA h g−1 at a 0.13 mA cm−2 current density, and the discharge capacity was 750 mA h g−1 after 10 cycles. In the process, NiP2 particles can be regarded as active materials and the other component is seen as a solid electrolyte.
Through electrodeposition, Xiang et al. synthesized porous nickel phosphide films. In the synthesis, polystyrene spheres could be assembled by them, and the many layers such a structure possessed were regarded as the template.62 When they got rid of the as-obtained template, spherical pores were reserved in the films. And the spherical pores were well-ordered as well as close-packed. Because of the existence of thin walls between the adjoining pores, a network nanostructure that was three-dimensional was obtained, and in this way, lithium ion transfer through the passivating layer is faster than before. What is more, this sort of nanostructure could stimulate charge transfer which enters into the fabricated network nanostructure. The two explanations above are based on electrochemical impedance spectra (EIS).
Hence, the network nanostructure delivers a momentous improvement in the electrochemical performance (a schematic illustration regarding the stacking of pores in films is shown in Fig. 1), particularly giving a superior rate capability. And the capacity of these kinds of triple-layer Ni3P porous films still respectively is maintained at 557 mA h g−1 and 243 mA h g−1 at a charge–discharge rate of 0.2C and 5C (1C = 388 mA g−1) after 50 cycles. While the discharge–charge rate rises 25 times, the reversible capacity remains 44%. These electrochemical performances are much more excellent than the electrochemical performances of single or double layer films.
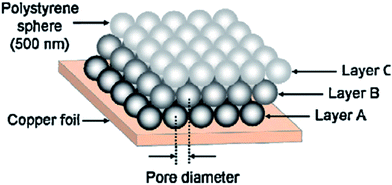 | ||
| Fig. 1 Schematic illustration regarding the stacking of pores in films. Adapted with permission from ref. 62, © 2011 Elsevier. | ||
An advanced Ni3P–Ni array, synthesized using electroless deposition, which was on a three-dimension nickel foam was utilized as an electrode in LIBs by Liu et al.104 The array structure of Ni3P–Ni can not only adapt to volume changes in the process of lithiation/de-lithiation but can also accelerate the high-rate capability, in that the interstices can serve as an ideal buffer for volume expansion in such structures. The Ni3P–Ni arrays showed excellent electrochemical performance and were used as anodes for LIBs, possessing excellent rate capability as well as a specific capacity of 360 mA h g−1 at a 0.02 mA cm−2 current density after 100 cycles.
Nickel phosphide films were prepared by Lu et al. through electrodeposition. At room temperature, they electrodeposited four genres of Ni films by utilizing a liquid which was composed of choline chloride and ethylene glycol.61 They obtained Ni films whose morphology was sheet-like when the concentration of Ni2+ was 0.5 M, while they also obtained Ni films whose morphology was sphere-like when the concentration of Ni2+ was 1.0 M. As a consequence, the surface morphologies of these Ni–P films are related to the initial nickel films.
As for the Ni–P films, different phases, which were Ni12P5 and Ni2P, could be obtained through phosphorization. Then they investigated the electrochemical performance of these Ni–P films, which serve as anodes in LIBs. Cyclic voltammetry as well as galvanostatic charge–discharge tests manifested that their electrochemical performance heavily relied on the surface morphology as well as the thin-film thickness. The as-prepared Ni2P films displayed excellent electrochemical performance, including a 398.5 mA h g−1 reversible discharge capacity. In addition, the samples possessed 91.4% retention after 50 cycles.
A composite with Ni12P5 nanoparticles encapsulated in carbon fiber was proposed by Zhang et al.105 And it should be especially noted that the composite is peapod-like. In the synthesis, the precursors are NiNH4PO4 nanorods; in addition, glucose could work as a carbon source. Fig. 2 shows the whole synthetic process and TEM images display the characteristic structure of the peapod-like composite in Fig. 3a and b. Due to the hydrogen bonding which exists between carbon and the precursor, a polymer layer could be hydrothermally synthesized and later transformed into carbon fiber through the method of inert calcination. The NiNH4PO4 nanorods simultaneously turned into Ni12P5 nanoparticles that were encapsulated in carbon fiber. In the whole process, they suffer from decomposition as well as reduction, which is on account of the high temperature as well as the carbon fibers.
 | ||
| Fig. 2 Synthetic process for a peapod-like composite with Ni12P5 nanoparticles encapsulated in carbon fiber. Adapted with permission from ref. 105, © 2014 Wiley. | ||
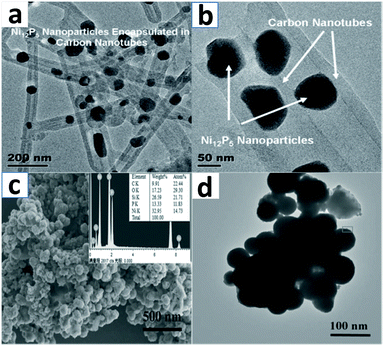 | ||
| Fig. 3 TEM images display typical structures relating to the peapod-like composite. The resolution increases from (a) to (b), little by little. (c) An SEM image of Ni5P4/C spheres. (d) A TEM image of the corresponding composite of the Ni5P4/C spheres. (a, b) Adapted with permission from ref. 105, © 2014 Wiley; (c, d) ref. 48, © 2012 Wiley. | ||
The synthesized composite delivered excellent performance when it was used as the anode in LIBs, compared with materials which are pure phase. To be specific, over 200 cycles, a specific capacity could be attained of 600 mA h g−1. And the improved performance was attributed to synergistic effects, which are associated with the combination of Ni12P5 nanoparticles and carbon fiber to some extent.
Lu et al. synthesized a single-phase Ni5P4/C composite.48 In the synthesis, they utilized a thin carbon shell, and they realized the synthesis though a two-step method which consists of a wet-chemistry reaction as well as a solid-state reaction. And the reason for the transformation from binary phase Ni5P4–Ni2P to single-phase Ni5P4 is that phosphorus atoms diffuse in the carbon shells when the solid-state reaction is in progress. Fig. 3c and d show an SEM image of the Ni5P4/C spheres as well as a TEM image of the corresponding composite of the Ni5P4/C spheres.
And in addition, galvanostatic charge–discharge measurements manifest that the as-prepared Ni5P4/C composite delivers superior rate capacity as well as excellent cycling stability. And almost 76.6% of the second capacity (644.1 mA h g−1) can be preserved after 50 cycles at a rate of 0.1C. Besides, at a high rate of 3C, the specific capacity of Ni5P4/C can reach 357.1 mA h g−1. Generally, carbon shells which are amorphous can reinforce properties of the composite, such as conductivity. In addition, the aggregation of active particles can be restrained. In this way, structural stability as well as reversibility during cycling can be realized.
Bai et al. synthesized a peapod-like Ni2P/C composite.65 They utilized NiNH4PO4H2O nanorods and these can be used as templates. Because of their enriched porosity as well as large active surface areas (113.7 m2 g−1), peapod-like composites deliver excellent performance when utilized as anodes in LIBs and in HER catalysts (as is shown in Fig. 4). When utilized as an anode in LIBs, the prepared nanocomposite showed 632 mA h g−1 capacity at 0.1 A g−1. What is more, they also delivered excellent rate capabilities when possessing a capacity of 439 mA h g−1, even at 3 A g−1 current density. And their capacity can reach as high as 628 mA h g−1, with a pretty long cycle life, as shown in Fig. 5a.
 | ||
| Fig. 4 The schematic diagrams illustrate the synthetic process for peapod-like Ni2P/C nanocomposites. Adapted with permission from ref. 65, © 2015 the Royal Society of Chemistry. | ||
 | ||
| Fig. 5 (a) Electrochemical performances of peapod-like Ni2P/C composites: cycling performance and coulombic efficiency within a voltage range of 0.01–3.0 V vs. Li/Li+ at 0.1 A g−1; (b) SEM images of Ni2P nanosheets; (c) comparison of Cu3P with Li4Ti5O12 and graphite according to charge/discharge potential, specific capacity, and specific energy density, as well as volumetric energy density. (a) Adapted with permission from ref. 65, © 2015 the Royal Society of Chemistry; (b) ref. 106, © 2012 the Royal Society of Chemistry; (c) ref. 108, © 2014 the Royal Society of Chemistry. | ||
Lu et al. synthesized porous Ni2P nanosheets through an organometallic method without a hitch.106 And they succeeded in realizing a synthesis using nickel nanosheets as a template, and the morphological characterization of the Ni2P nanosheets is displayed in Fig. 5b. These Ni2P nanosheets possess a pretty thin thickness that is around 3 nm, as well as a high Brunauer–Emmett–Teller (BET) surface area owing to the highly porous structure, which could result in good lithium storage capacity as well as excellent rate capability. What is important is that the diffusion length regarding the lithium ions will be short due to the thin and porous Ni2P sheets. These nanosheets, when utilized as anodes in LIBs, exhibit 379.8 mA h g−1 reversible discharge capacity after 50 cycles and an excellent rate capability.
Lu et al. successfully fabricated di-nickel phosphide (Ni2P) nanowires which are single crystalline.78 And these nanowires possessed an 8 nm uniform diameter as well as 100–200 nm lengths. They were synthesized via thermal decomposition in which the continuous delivery of Ni–TOP complexes is necessary. Fig. 9c shows a TEM image of the Ni2P nanowires. Compared to the hexangularly structured Ni2P nanoparticles, these Ni2P nanowires display specific reversible capacities of 434 mA h g−1 at 0.1C and 326 mA h g−1 at 0.5C after 50 cycles; in addition, the samples show excellent rate performance. Thus performances have been largely improved and the reasons for the improvement are that the size is small and the cylindrical structure is stable. And it made the interfacial contact area with electrolytes especially high; in addition, it could also relieve the strain and adapt to the volume expansion/contraction of the Ni2P nanowires. What is more, as is shown in electrochemical impedance spectra (EIS), the enhanced electrical conductivity of these nanowires could promote the transportation of lithium ion as well as electrons in the electrode.
Lu et al. successfully synthesized hierarchical nanostructured nickel phosphide (h-Ni2P) spheres.64 A one-pot reaction involving an organic phase strategy is utilized by them, and in the synthetic process, the solution should be continually stirred. And the precursor they used was nickel acetylacetonate, and trioctylphosphine (TOP) can be used as the phosphorus source. Among the organic-phase mixture, a surfactant is necessary, and tri-n-octylamine as well as oleylamine (OAm) can be utilized. The specific process is shown in Fig. 6.
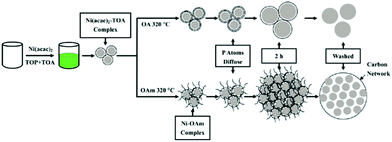 | ||
| Fig. 6 Schematic illustration of the fabrication of h-Ni2P. Adapted with permission from ref. 64, © 2011 American Chemical Society. | ||
The h-Ni2P spheres, which consist of ordered nanoparticles of 5–10 nm size as well as being filled with amorphous carbon, deliver a superior cycling performance to the Ni2P spheres, which are synthesized by utilizing oleic acid (OA), which is regarded as the surfactant. The contact area, which is between electrolytes and Ni2P, could increase owing to the hierarchical structure, and this is because such a structure could offer more sites to accommodate Li+ and shorten the length for Liz+ diffusion, as well as enhance the reaction reactivity. Furthermore, it is the amorphous carbon as well as the hierarchical nanostructures that could adapt to expansion in volume and in this way the stability of the electrodes increases during cycling. The prepared h-Ni2P electrode delivers excellent capacity and Coulombic efficiency. After 50 cycles, the reversible capacity of the h-Ni2P spheres is as high as 365.3 mA h g−1 at 0.5C, and 257.8 mA h g−1 at 1C. And at a high rate of 3C, the specific capacity of h-Ni2P is 167.1 mA h g−1.
Stan et al. synthesized a Cu3P sample through a mechanochemical method (ball milling) when n-dodecane was present.56 Then the favourable electrochemical properties of the Cu3P sample were presented, and the Cu3P sample showed an almost theoretical specific capacity when charged to 0.02 V for the first cycle. But the capacity faded as cycling was prolonged. When the sample was cycled over the voltage range from 2.0 to 0.50 V (vs. Li/Li+), the performance improved. The types of formed products result in a capacity decline when cycled to a lower cut-off of 0.02 V. And the types are connected with the energy barrier, which can be used to reconvert to primary materials. However, when the potential is 0.50 V, the energy barrier can be much smaller relatively, and what is more, capacity retention can also reveal it.
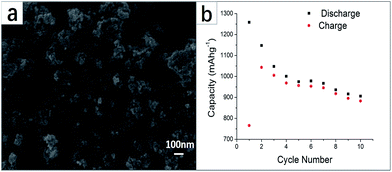 | ||
| Fig. 7 (a) SEM image of amorphous FeP2 synthesized at low temperature; (b) discharge and charge capacities for the first 10 cycles of amorphous FeP2 at 0.1C. Adapted with permission from ref. 66, © 2012 American Chemical Society. | ||
At room temperature, Chandrasekar et al. prepared iron phosphide over a Cu substrate at high temperature.60 A cost-effective electrodeposition method was utilized by them to synthesize samples, and aqueous acidic electrolyte was utilized in the synthetic process. The phosphorus content of the alloys differed and varied with the composition of the source. And as-prepared deposits were annealed at 400 °C for 3 h. Such a process goes on under a constant inert gas flow rate in a tubular furnace. Fig. 9d shows an FEGSEM image of electrodeposited Fe2P annealed samples. Then they characterized the phase composition as well as the morphology by utilizing X-ray diffraction and scanning electron microscopy. They tested samples when the samples were used as anode materials between 0.01 and 2.5 V, at a constant rate of 10 μA cm−2.
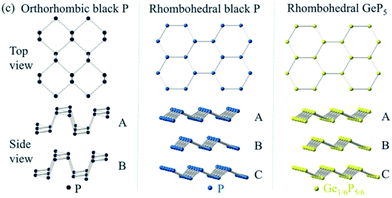 | ||
| Fig. 8 Crystal structures of orthorhombic black P, rhombohedral black P and rhombohedral GeP5. Adapted with permission from ref. 55, © 2015 the Royal Society of Chemistry. | ||
 | ||
| Fig. 9 (a) and (b) SEM images of 10–10 h milled GeP5/C nanocomposites; (c) TEM image of Ni2P nanowires; (d) FEGSEM image of electrodeposited Fe2P annealed samples. (a, b) Adapted with permission from ref. 55, © 2015 the Royal Society of Chemistry; (c) ref. 78, © 2012 the Royal Society of Chemistry; (d) ref. 60, © 2014 Springer. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 10 times that of the conductivity of black P and graphite.
000 and 10 times that of the conductivity of black P and graphite.When used as an anode, GeP5 and the carbon composite both show an excellent reversible capacity of 2300 mA h g−1, as well as a good initial coulombic efficiency of about 95%. What is more, ex situ XRD as well as CV tests show GeP5 goes through conversion as well as a lithium storage mechanism, which is connected with the alloy. Furthermore, both germanium and phosphorus make a contribution to the capacity. What is more, GeP5/C delivers excellent cycle stability, as well as possessing a high rate capacity of 2127 mA h g−1 at 5 A g−1.
Besides, their excellent cycling stability as well as rate performance have been characterized acting as anodes in LIBs. Results display that Sn4P3 nanoparticles of the smallest size could deliver the greatest cycling as well as rate performances. These nanoparticles exhibited a discharge capacity that was about 612 mA h g−1 after 10 cycles, in addition, they could retain 442 mA h g−1 after 320 cycles at 100 mA g−1 within a voltage range of 0.01–3.0 V. Because of their small size, the performances of the Sn4P3 electrodes might be improved. What is more, the as-obtained Sn4P3 nanoparticles have been characterized as anodes for Na-ion batteries (NIBs) as well, and could exhibit a 305 mA h g−1 reversible capacity after 10 cycles at a 50 mA g−1 current density.
3.2 Sodium storage – Na-ion batteries
LIBs have received much attention owing to their cycling stability, excellent energy density and many other advantages; these have made LIBs universally utilized as rechargeable power sources in portable electronic devices, HEVs and EVs.109–111 What is more, there are lots of new electrode materials emerging so as to improve the performance of LIBs. Nevertheless, the high expense and insufficient abundance of lithium resources hinder the development of LIBs. It is difficult to satisfy need for large-scale application, especially in industry.112–115Na-ion batteries (NIBs) have received considerable attention as a potentially alternative to LIBs, with readily available and geographically diverse reserves of the metal.116–123 Hence, the cost of sodium is lower than that of lithium and there is also the potential for cost reduction via electrodes.124–129 However, it is a challenging task to develop a suitable and excellent host electrode material for accommodating sodium ions which allows reversible ion insertion and rapid extraction in its structure, owing to the around 55% larger radius of Na+ compared with Li+, which causes slower kinetics with Na+.130–134
Although there exists not too much research based on TMPNs in the field of NIBs, in recent times, with research into electrode materials for NIBs, TMPNs have gradually emerged and turned into research hot points. Below are recent developments in NIBs, with specific electrode materials and their electrochemical performances. For example, the performances of some materials are summarized in Table 2.
 | ||
| Fig. 10 (a) A schematic illustration providing information on uniform yolk–shell Sn4P3@C nanosphere anodes; (b) schematic illustration of the sodiation process in various Sn4P3-based anodes including bare Sn4P3 nanoparticles and core–shell Sn4P3@C nanoparticles, as well as the current yolk–shell Sn4P3@C nanoparticles. Adapted with permission from ref. 63, © 2015 the Royal Society of Chemistry. | ||
Fig. 11a and b exhibit high-magnification SEM and low-magnification TEM images of the final products. The fabricated structure of the Sn4P3 electrodes is very crucial. A carbon shell that is thin and conformal, as well as self-supporting, can thoroughly protect the yolk–shell Sn4P3 nanoparticles. Because of the void space between the shells and nanoparticles, the expansion of Sn4P3 can be carried out without deforming the shells or disrupting the stable solid-electrolyte interphase (SEI) which is located on the outside surface. Because of the unique structure, the as-prepared nanospheres, when used as anodes in NIBs, deliver an excellent reversible capacity of 790 mA h g−1 and great rate capability, as well as cycling stability (a highly stable capacity of 360 mA h g−1 at 1.5C after a long-term 400 cycles).
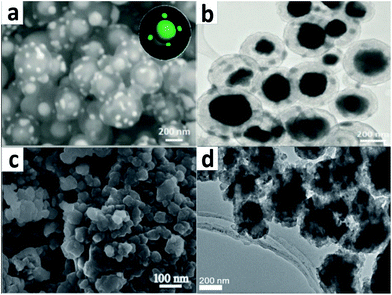 | ||
| Fig. 11 (a) High-magnification SEM and (b) low-magnification TEM images of uniform yolk–shell Sn4P3@C nanosphere products; (c) SEM image of CoP particles; (d) HRTEM image of a Sn4P3/C composite. (a, b) Adapted with permission from ref. 63, © 2015 the Royal Society of Chemistry; (c) ref. 59, © 2015 Elsevier; (d) ref. 135, © 2014 American Chemical Society. | ||
Qian et al. utilized a synthetic approach for a Sn4P3/C nanocomposite with good performance that can serve as the anode in NIBs.135 A HRTEM image of Sn4P3/C is shown in Fig. 11d. The as-obtained anodes show an excellent reversible capacity of 850 mA h g−1 as well as superior rate capability, with 50% capacity output at 500 mA g−1. Besides, good cycling stability, with 86% capacity retention over 150 cycles, is demonstrated. The remarkable performance derives from a synergistic Na-storage mechanism in the Sn4P3 anodes, as shown in Fig. 12.
 | ||
| Fig. 12 Schematic illustration of the Na-storage mechanism in Sn4P3 electrodes. Adapted with permission from ref. 135, © 2014 American Chemical Society. | ||
Kim et al. prepared tin phosphide (Sn4P3) through ball milling.57 When tested as anodes in NIBs, Sn4P3 delivered excellent electrochemical performances, for example, a reversible capacity of 718 mA h g−1. In addition, the cycling stability is excellent and the decay in capacity is so negligible that it might be neglected over 100 cycles. As is shown in Fig. 13a, Sn4P3 delivered a superior cycling performance to Sn, although both electrodes showed a similar reversible capacity of around 718 mA h g−1 when a fluoroethylene carbonate (FEC) additive was utilized. The sodiation profile of Sn4P3 exhibits two steps situated at ∼0.2 and 0.01 V vs. Na/Na+, and this can be further supported through differential capacity (dQ/dV) plots (Fig. 13b).
 | ||
| Fig. 13 (a) Cycling performances of Sn4P3 and Sn electrodes obtained with and without FEC additive. (b) Differential capacity (dQ/dV) plots for Sn4P3 electrodes over the first cycle, with and without FEC additive. Adapted with permission from ref. 57, © 2014 Wiley. | ||
Through ball milling between elemental phosphorus and tin powder, a Sn4+xP3@(Sn–P) composite was prepared by Li et al. in large quantities.58 Fig. 14a and b show SEM and HRTEM images of the Sn4+xP3@(Sn–P) composite. The electrochemical performance of the composite was examined through use as an anode in NIBs. The results showed that a Sn4+xP3@(Sn–P) electrode with a CMC binder showed a high capacity of 502 mA h g−1 at a current density of 100 mA g−1. Besides, the cycling stability of the Sn4+xP3@(Sn–P) electrode was improved because of the addition of 5% fluoroethylene carbonate (FEC) to the electrolyte. What is more, the as-prepared anode exhibited a 465 mA h g−1 stable capacity, whose retention was 92.6% at a current density of 100 mA g−1 over 100 cycles. This capacity is excellent for anodes in NIBs. Furthermore, the as-obtained anode delivered great rate capability.
 | ||
| Fig. 14 (a) SEM and (b) HRTEM images of a Sn4+xP3@(Sn–P) composite. The inset in (b) is the SAED pattern from lower magnification TEM; (c) SEM image; (d) TEM image. (a, b) Adapted with permission from ref. 58, © 2014 Wiley; (b) ref. 31, © 2015 Elsevier; (c, d) ref. 136, © 2015 the Royal Society of Chemistry. | ||
 | ||
| Fig. 15 A schematic illustration of the formation process for a CuP2/C composite nanostructure and the structural evolution during electrochemical charging and discharging. Adapted with permission from ref. 136, © 2015 the Royal Society of Chemistry. | ||
Several studies of the CuP2/C composites, including an SEM image and TEM image, are shown in Fig. 14c and d. As a potential anode candidate, the as-obtained samples could reach the goal of fast reversible sodiation as well as desodiation. And the realization of this function relies on the mechanism of conversion. In addition, they exhibited a great capacity of more than 500 mA h g−1. The excellent rate capability as well as the short-term cycling stability are other advantages for these samples.
NiP3-based electrodes were utilized as negative electrodes for NIBs. The fabrication of a composition close to Li3P as well as Na3P was revealed during research regarding the reaction mechanism. In the fabrication, they embedded nickel nanoparticles to synthesize the target samples after discharge. Moreover, owing to the fabrication of a carboxymethyl cellulose/carbon black (CMC/CB) electrode, NiP3 electrodes deliver pretty excellent capacity. And for NIBs, the reversible capacity can reach in excess of 900 mA h g−1 after 15 cycles.
3.3 Supercapacitors
Among numerous ways of energy storage, supercapacitors (SCs) have been recognized as a promising kind of energy storage device and have attracted more and more attention owing to higher power densities, faster charging/discharging rates, and longer cyclic performances, as well as better safety compared to any other energy storage strategies, such as LIBs and NIBs.138–152SCs can be generally divided into two sorts, (i) electrical double-layer capacitors (EDLCs) and (ii) pseudocapacitors (PDCs), based on the energy storage mechanism. The former stores energy in a non-faradic way through the accumulation of charge at the interface between the electrode and electrolyte, while the latter is based on reversible faradic reactions that lead to pseudocapacitive behavior.153–161
In general, an ideal electrode material for SCs should possess high conductivity, high energy and power delivery, a pretty large surface area, and suitable pore size distribution, as well as excellent cycling ability and electrochemical reproducibility.162,163 However, the practical application of SCs is restricted by the lack of electrode materials with facile synthetic methods as well as low cost. Thus, searching for excellent electrode materials was the focus in past decades. Inchoate studies of SCs primarily concentrated on carbonaceous materials, and carbonaceous materials possessed relatively low specific capacitance as well as instability when at a relatively high charge–discharge rate.164 And their energy density is lower than that of rechargeable batteries, which hinders the utilization of SCs.165–168
Thanks to recent remarkable progress, TMPNs are noted to have metalloid properties as well as excellent electrical conductivity, so they are fit to be electrode materials.169–173 Among all the TMPNs, nickel phosphide nanomaterials have drawn extensive interest as they include a number of phases and excellent properties, as well as potential electrochemical applications.174 The following examples are detailed statements of recent research based on nickel phosphide nanomaterials.
An effective phosphorization synthesis was demonstrated to strengthen supercapacitor performance, based on transition metal oxides, as well as hydroxides, in a study operated by Zhou et al.175 Fig. 16a and b show SEM images of Ni2P NS/NF. Through the phosphorization of Ni(OH)2 nanosheets, a three-dimensional networked Ni2P nanosheet array can be grown on a surface which consists of Ni foam. According to the voltammetric measurements, Ni foam-supported Ni2P nanosheets (Ni2P NS/NF) could be used as electrodes and they were recognized to show a specific capacitance of 2141 F g−1 at 50 mV s−1 (Fig. 16c) and could remain at 1109 F g−1 even at a current density of 83.3 A g−1. In addition, the specific capacitance could remain almost steady at 1437 F g−1 between 2000 and 5000 charging/discharging cycles (Fig. 16d). They found that similar performance can be observed in Ni2P powder, because it has eliminated the effect of the Ni foam.
 | ||
| Fig. 16 (a) and (b) SEM images of Ni2P NS/NF; (c) CV curves for Ni2P NS/NF at different scan rates. (d) Cycling stability of Ni2P NS/NF and Ni(OH)2 NS/NF at a current density of 10 A g−1. Adapted with permission from ref. 175, © 2015 Wiley. | ||
Lu et al. successfully coated Ni2P particles via an electroless plating method homogenously with amorphous Ni.176 Fig. 17a and b show an SEM image and TEM image of the Ni2P/Ni composite, which would be created through an electroless plating process for 10 min. The Ni2P nanoparticles are completely coated with a 20–30 nm thick layer. The pseudocapacitive behavior regarding the as-prepared samples can be characterized using cyclic voltammograms as well as galvanostatic charge–discharge tests in 2 M LiOH.
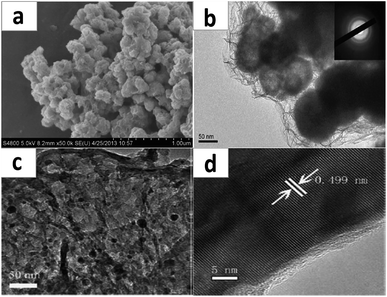 | ||
| Fig. 17 (a) SEM image of Ni2P/Ni; (b) TEM image of Ni2P/Ni; (c) TEM image and (d) HR-TEM of a Ni2P/rGO composite. (a, b) Adapted with permission from ref. 176, © 2013 the Royal Society of Chemistry; (c, d) ref. 79, © 2013 the Royal Society of Chemistry. | ||
It could be designated that the specific capacitance of the Ni2P/Ni composite decreased by 19%, from 581 F g−1 at 1 A g−1 to 464 F g−1 at 40 A g−1. What is more, the as-prepared samples also show excellent cycling capacity. The capacitance of the as-prepared samples is excellent. The specific capacitance can reach 1115 F g−1 at 2 A g−1, and it can be retained at 1029 F g−1 (92.3% capacity retention) after 3000 cycles. Importantly, the great improvement in pseudocapacitive performance could be attributed to the flask-like Ni coating. The flake-like coating could offer the fast transfer of ions and electrons, as well as a number of active sites.
An et al. successfully synthesized Ni2P nanoparticles on reduced graphene oxide (rGO) via a solid state reaction at low-temperature.79 TEM and HR-TEM images of the Ni2P/rGO composite are shown in Fig. 17c and d. And the Ni2P/rGO nanoparticles were utilized as a pseudocapacitive material, which is associated with energy storage. In addition, the specific capacitance of the as-prepared Ni2P/rGO is 2266 F g−1 and it also displays excellent cycling performance when utilized as a capacitor material. After 2500 cycles, the loss in specific capacitance is rather small and even negligible, as shown in Fig. 5d. This great result suggests that a Ni2P/rGO electrode is a candidate for a pseudocapacitor with excellent performance, such as superior specific capacitance as well as a stable cycling capacity.
Duan et al. proposed a novel synthetic method for the fabrication of core/shell nanostructures which are composed of two parts: noble metal cores, as well as semiconductor shells which are single-crystal and possess disparate crystal systems.177 And the synthetic route involves a simple phosphorization process, in which triphenylphosphine served as a capping agent as well as the phosphorous source which reacts with Au/Ni12P5 core/shell nanoparticles (NPs), and this process makes Au/Ni12P5 core/shell nanoparticles (NPs) convert from Au–Ni bimetallic heterodimers. The transformation process is illustrated in Fig. 18.
 | ||
| Fig. 18 Schematic illustration of the fabrication of Au/Ni12P5 core/shell NPs. Adapted with permission from ref. 177, © 2014 Nature. | ||
In addition, they further investigated the SC performance of the Au-modified Ni12P5 nanostructures. When serving as supercapacitor electrodes, the metal/semiconductor nanostructure with synergistic effects is superior to the oligomer-like counterpart. The specific capacitance of the electrode composed of core/shell nanoparticles (NPs) is up to 806.1 F g−1 (91.1% capacity retention after 500 charge–discharge cycles).
A one-step strategy has been utilized to prepare Ni2P nanoparticles. And these nanoparticles could be further embellished with graphene, and in this way graphene-modified Ni2P nanocomposites could come into formation. Du et al. tested the electrochemical performance of Ni2P samples.76 This showed that supercapacitor electrodes composed of the as-prepared samples delivered excellent electrochemical performance.
The as-obtained electrodes deliver a larger specific capacitance, better cycling stabilization and superior rate capability, as well as greater energy density. With only 5% (wt%) graphene loaded, the electrochemical performance could be greatly improved. Fig. 19b displays CV curves at different scan rates from supercapacitor electrodes composed of a Ni2P@5% GR(graphene) nanocomposite and a TEM image of the as-prepared Ni2P@5% GR (graphene) nanocomposite is shown in Fig. 19a. The improvement in the electrochemical performance is derived from the network structure. And such a structure allows for efficient charge migration as well as electrolyte diffusion, and furthermore it holds back the expansion/contraction of the volume as well as the corrosion of the Ni2P particles.
 | ||
| Fig. 19 (a) An SEM image of a Ni2P@5% GR nanocomposite; (b) CV curves at different scan rates recorded for supercapacitor electrodes consisting of a Ni2P@5% GR (graphene) nanocomposite. Adapted with permission from ref. 76, © 2014 Elsevier. | ||
Du et al. successfully synthesized nickel phosphide (NiP) nanoparticles using a one-pot solvothermal strategy that has been applied extensively.178 The obtained nickel phosphide was further assembled into two kinds of SCs: one is asymmetric and another is symmetric. They tested the electrochemical performance of these as-obtained assembled SCs in an electrolyte composed of 3.0 mol L−1 KOH. It turned out that the as-prepared asymmetric supercapacitors made of Ni2P nanoparticles as well as activated carbon (Ni2P//AC ASCs) delivered excellent electrochemical performance, such as a stable electrochemical window of 0–1.5 V, a superior energy density of 64.6 W h kg−1 at a power density of 1029 W kg−1, and great cycling stability (95.1% capacitance retention). And the specific results are shown in Fig. 20.
 | ||
| Fig. 20 (a) CV curves for Ni2P//AC ASCs at different scan rates; (b) discharge curves for the Ni2P//AC ASCs; (c) Ragone plot relating to the energy and power densities of Ni2P//AC ASCs; (d) cycling performance curves for Ni2P//AC ASCs and Ni2P//Ni2P SSCs. Adapted with permission from ref. 178, © 2015 Elsevier. | ||
On the contrary, the symmetric supercapacitors composed of Ni2P nanoparticles delivered an electrochemical performance that was not so good. It should be noted that Ni2P//AC ASCs could drive a number of electronic units by charging and then discharging. In addition, two asymmetric supercapacitors can be linked in series to drive a red light-emitting diode and a rotating motor.
4. Electrochemical energy conversion – solar cells
Solar energy conversion can be recognized as the most dependable and feasible approach to satisfy growing energy demands in an environmentally friendly way, and optimization regarding solar energy resources is momentous and indispensable.179 Dye-sensitized solar cells (DSSCs), as a 3rd generation photovoltaic (PV) conversion method, have been demonstrated to achieve conversion from solar energy to electricity,180,181 and what is more, DSSCs possess the strengths of low-cost, high conversion efficiency and easy fabrication.182–187 Since the pioneering work that was reported by O'Regan and Grätzel, quantities of papers have been published to put forward techniques and novel materials for the implementation of DSSCs.188–192In DSSCs, the counter electrode (CE) is the pivotal component affecting the photovoltaic properties of DSSCs, and it can be utilized as a mediator which can collect electrons to reduce I3− to I− to regenerate the redox couple.193,194 And it generally consists of platinum because of its favourable electrocatalytic activity for the iodide–triiodide redox couple as well as its great conductivity.195–197
But the mass production of DSSCs is restricted by the high cost and corrosion of platinum.198 The known reserves of platinum all over the world are rather limited. Meanwhile, in recent years, the development of proton exchange membrane fuel cells (PEMFCs) has aggravated the shortage of platinum resources, as in PEMFCs platinum can be utilized as an indispensable electrocatalyst.71
Hence, there is a desperate need to exploit low-cost as well as high-efficiency counter electrodes possessing high electrocatalytic activity that are analogous to platinum and, in this way, the traditional and exorbitant Pt counter electrode can be replaced in DSSCs. And it should be noted that TMPNs are practical alternatives owing to their excellent electrocatalytic activity, superior electrical conductivity, and great stability.
4.1 Nickel phosphide materials
At 180 °C, a hydrothermal reaction gives access to the synthesis of nickel phosphide-embedded graphene, which is in an aqueous solution of ethylene glycol.74 The hydrothermal synthesis involves red phosphorus, nickel chloride and graphene oxide. And the prepared samples were studied as counter electrodes in dye-sensitized solar cells. It is emphasized that nickel phosphide-embedded graphene delivers superior catalytic activity and weak diffusion impedance. Fig. 21a displays I–V curves of DSSCs which possess different counter electrodes. | ||
| Fig. 21 (a) I–V characteristic curves of DSSCs with different counter electrodes consisting of graphene, Ni12P5, a graphene–Ni12P5 composite and FTO-Pt, measured under simulated sunlight of 100 mW cm−2 (AM 1.5); (b) CVs of Pt, Ni12P5, and Ni2P electrodes at a scan rate of 10 mV s−1 in acetonitrile containing 0.5 M LiClO4, 50 mM LiI and 10 mM I2. The inset shows consecutive CVs and changes in the redox peak currents with the cycle number for the Ni2P (PR) electrode; (c) the photocurrent–voltage (J–V) characteristics of DSSCs employing Pt, Ni2P (PR), Ni2P (PS), and Ni12P5 (PS) counter electrodes; (d) J–V curves for T2/T− based DSSCs using mesoporous carbon (MC), Ni5P4, Ni5P4/C and Pt counter electrodes. (a) Adapted with permission from ref. 74, © 2012 the Owner Societies; (b, c) ref. 199, © 2013 the Royal Society of Chemistry; (d) ref. 200, © 2012 the Royal Society of Chemistry. | ||
Through pulse-reverse deposition, Wu et al. directly coated a Ni2P nanolayer, which possesses porous nanospheres, on tin oxide glass doped with fluorine and this was investigated for use as a counter electrode catalyst in DSSCs.199 Periodic pulse-reverse (PR) deposition was fulfilled with a voltage of −0.8 V for 6 s and a reverse voltage of 0.1 V for 24 s, and the periodic voltage was repeated for 500 cycles. Compared with PR deposition, potentiostatic (PS) deposition was accomplished using a voltage of −0.8 V for 50 min.
The preparation strategy impacts on the morphology as well as the microstructure of the nickel phosphide electrodes. In fact, the differences in the as-obtained particle sizes are owing to the voltage which is applied in the process. And PS deposition results in the sequential growth of the grain, which forms bigger nanoparticles. On the contrary, PR deposition constrains the growth of the grain, owing to the anodic dissolution of regions which are rich in nickel during the anodic process, and is inclined to prepare smaller nanoparticles upon the surface made up of fluorine-doped tin oxide (FTO).
A nanolayer of nickel phosphide possessing porous nanospheres was synthesized through PR deposition. Fig. 22a displays an SEM image of the nickel phosphide serving as an electrode which is prepared through PR deposition. Fig. 22b displays a TEM image of the nickel phosphide nanoparticles which could be obtained using PR deposition after annealing at 500 °C for 1 h.
 | ||
| Fig. 22 (a) Top-view as well as side-view SEM images of nickel phosphide electrodes prepared using PR deposition; (b) TEM image of nickel phosphide nanoparticles obtained from PR deposition, scale bar = 50 nm; (c) SEM morphology image of Ni5P4/C; (d) SEM image of nickel phosphide/FTO. (a, b) Adapted with permission from ref. 199, © 2013 the Royal Society of Chemistry; (c) ref. 200, © 2012 the Royal Society of Chemistry; (d) ref. 201, © 2015 the Royal Society of Chemistry. | ||
The anodic dissolution in Ni-rich regions that follows the deposition of the cathode resulted in the formation of porous Ni2P nanospheres on the surface made up of fluorine-doped tin oxide (FTO) for PR deposition, while PS deposition could form a Ni12P5 layer with compact nanospheres. The transparency and catalytic activity of the Ni2P electrodes obtained using PR deposition are greater than those of the PS-deposited Ni12P5 electrodes.
Fig. 21c shows the J–V characteristics of DSSCs that employ platinum as well as nickel phosphide counter electrodes (CEs). The photovoltaic properties of DSSCs with Ni2P obtained using PR deposition can be comparable to the photovoltaic properties of DSSCs with platinum and, in addition, they are superior to the photovoltaic properties of DSSCs with Ni2P (PS) and Ni12P5 (PS). Fig. 21b shows cyclic voltammograms (CVs) of Ni2P (PR), Ni12P5 (PS), and platinum electrodes. The nickel phosphide nanoparticles display a distinctive electrocatalytic performance but resemble the Pt electrodes.
Wu et al. proposed that carbon-supported Ni5P4 (Ni5P4/C), molybdenum phosphide (MoP) and nickel phosphide (Ni5P4) can be used in DSSCs as counter electrode (CE) catalysts and they can be used for the reconstruction of the traditional I3−/I− redox couple and the novel organic T2/T− redox couple.200 With regard to the I3−/I− redox couple, MoP and Ni5P4 showed decent catalytic activity and DSSCs using MoP and Ni5P4 CEs produced power conversion efficiencies (PCEs) of 4.92 and 5.71%.
Similarly, when Ni5P4 and mesoporous carbon (MC) were integrated into one composite (Ni5P4/C), the catalytic activity could be enhanced significantly. Fig. 22c displays an SEM morphology image of Ni5P4/C, and DSSCs with Ni5P4/C delivered a 7.54% PCE, which verged on that of DSSCs utilizing platinum counter electrodes (7.76%). As is shown in Fig. 21d, the advantages of Ni5P4/C towards the T2/T− redox couple were more important than those towards the I3−/I− redox couple. Ni5P4/C showed much better performance than Pt and DSSCs with Ni5P4/C CE showed a PCE of 4.75%, much greater than the photovoltaic performance of DSSCs with a platinum CE (3.38%).
A monolayer of nickel phosphide clusters possessing mesoporous nanoparticles could be immediately synthesized on fluorine-doped tin oxide (FTO) glass by Wu et al. through cyclic voltammetric deposition.201 They used the prepared samples as CEs in DSSCs. Fig. 23 clearly illustrates the formation process of the nickel phosphide clusters, which are discrete on fluorine-doped tin oxide (FTO) glass, by methods of cyclic voltammetric deposition and an SEM image of nickel phosphide/FTO is shown in Fig. 22d. Cyclic voltammetry incorporated anodic dissolution in regions rich in nickel, which follows the deposition of the cathode, and it could give rise to the fabrication of discrete clusters possessing mesoporous nanoparticles.
 | ||
| Fig. 23 Schematic diagram illustrating the formation of discrete nickel phosphide clusters on fluorine-doped tin oxide (FTO) glass through cyclic voltammetric deposition. Adapted with permission from ref. 201, © 2015 the Royal Society of Chemistry. | ||
Nickel phosphide can be characterized as a specific substance, Ni5P4, through annealing at 500 °C, and furthermore, its electrocatalytic features could be assessed using cyclic voltammetry as well as the electrochemical impedance existing in the iodide/triiodide system. The mesoporous Ni5P4 catalyst synthesized using cyclic voltammetric methods displays excellent electrocatalytic ability towards the iodide/triiodide redox couple because of the low diffusion impedance and resistance to charge transfer. The PCE of DSSCs with a Ni5P4 counter electrode can reach 7.6%, which is higher than that of DSSCs using platinum nanocluster counter electrodes (7.2%).
4.2 Indium phosphide materials
Wang et al. proposed that a graphene based van der Waals heterostructure has superior performance, especially for a graphene/semiconductor Schottky junction.202 And it offers a new platform for solar cell applications.203,204 Compared with Si, which is the dominant material in current photovoltaic applications,205–207 indium phosphide (InP) has a bandgap of 1.34 eV, which is located in an optimum energy range for solar energy conversion. The band diagram of a graphene/p-InP Schottky junction is shown in Fig. 24a. | ||
| Fig. 24 (a) Band diagram of the graphene/p-InP Schottky junction interface showing the tunable graphene Fermi level EF-gr; (b) schematic structure of the graphene/p-InP Schottky junction device. Adapted with permission from ref. 202, © 2015 Elsevier. | ||
They achieved graphene/p-InP solar cells possessing a power conversion efficiency (PCE) of 3.3% under AM 1.5 G illumination through delicately designing and engineering a van der Waals heterostructure between graphene and indium phosphide (InP), which had a suitable bandgap of 1.34 eV for solar energy conversion. Fig. 24b shows the schematic structure of the graphene/p-InP Schottky junction device. Chemical doping or electrical field modulation was used to tune the Fermi level of graphene, which led to a PCE of 5.6% for the device under gating effects. Separation and recombination processes in the graphene/InP heterojunction, without and with graphene doping, are displayed in Fig. 25a and b, respectively. Furthermore, according to transient photoluminescence measurements, the interface recombination rate could be reduced while graphene is doped or gated.
 | ||
| Fig. 25 Schematic diagrams of the carrier separation and recombination processes in graphene/InP (a) and doped graphene/InP (b) heterojunctions after excitation from a light source. Adapted with permission from ref. 202, © 2015 Elsevier. | ||
Zheng et al. investigated solar cells that were based on InP, which were synthesized via non epitaxial thin-film vapor–liquid–solid (TF-VLS) synthetic strategies.80 There exist a molybdenum back contact, p-InP absorber layer, n-TiO2 electron selective contact, and indium tin oxide transparent top electrode in the cell structure.
In addition, they introduced an ex situ p-doping process for TF-VLS grown InP and examined the performance of the solar cells, for example, the optoelectronic uniformity as well as the electrical behavior of grain boundaries. Under simulated 1 sun illumination, the PCE of first generation solar cells can reach 12.1% with an open-circuit voltage (VOC) of 692 mV, a short-circuit current (JSC) of 26.9 mA cm−2, and a fill factor (FF) of 65%. Although limited by series resistances, including the top contact, the FF of cells can be alleviated in the future via an optimization of the devices. Under 1 sun, the highest measured VOC was 692 mV, and this approached the optically implied VOC of around 795 mV, which can be obtained from the luminescence yield of p-InP.
Ko et al. fabricated indium phosphide (InP) nanopillars which were single-crystalline and grown on a substrate composed of silicon.208 They synthesized the samples for solar cells and they provided an excellent performance, with a 0.534 V VOC under Air Mass 1.5 Global (AM 1.5 G) illumination. What is more, the PCE can reach 19.6%.
The samples were utilized for solar cells and then they were tested using a solar simulator. The I–V characteristic curves which could be tested in the dark are shown in Fig. 26. Illuminated using a AM 1.5 G solar spectrum, the as-prepared solar cells delivered a VOC of 0.534 V and an ISC of 96.0 pA, as well as a fill factor of 48.2%. Normalizing ISC to the projected exposed area yields a JSC of 76.3 mA cm−2. This is more than a factor of 2 higher than the JSC of 32.2 mA cm−2 predicted using the Shockley–Queisser limit for a planar solar cell.
 | ||
| Fig. 26 Single nanopillar solar cell electrical characteristics. (a, b) Room-temperature dark and 1 sun (AM 1.5 G) IV characteristics of a single InP nanopillar solar cell on the linear (a) and log (b) scale. Adapted with permission from ref. 208, © 2015 American Chemical Society. | ||
4.3 Gallium phosphide materials
Feifel et al. investigated n-Si/p-Si homojunction solar cells in which GaP served as a passivator as well as a contact layer on the n-Si emitter, with different approaches for backside passivation as shown in Fig. 27b.209 What's more, the backside structure of the final solar cell is pivotal to the structure of GaP/Si solar cells. They discussed disparate silicon solar cells possessing a GaP window, as well as contact layers which were grown using metal-organic vapor phase epitaxy (MOVPE). In addition, they established a process which was propitious for offering low resistance contacts because it could retain the quality of the bulk material silicon as well as offer backside passivation. | ||
| Fig. 27 (a) Illuminated measurements which are under an AM 1.5 G spectrum and an irradiation intensity of 1000 W m−2 and the dark I–V characteristics of an average type II solar cell. (b) The structure of the two different species of solar cells (left: type I, right: type II) that were produced and characterized. Adapted with permission from ref. 209, © 2015 IEEE. | ||
The two backside passivation approaches that could be applied prior to the metal-organic vapor phase epitaxy (MOVPE) process are characterized as type I and type II. Type I, which is based on the strength of a highly doped BSF layer, and passivation of type II, composed of an Al2O3 layer, were compared. The cells with Al2O3 backside passivation deliver higher VOC values compared with the BSF cells, and type II solar cells deliver a VOC of 633 mV and a JSC of 24.7 mA cm−2 without an antireflective coating, as well as a FF of 79.9%. In addition, the I–V curve under illumination and a dark I–V of an average type II cell is shown in Fig. 27a.
Hybrid organic–inorganic solar cells were fabricated by Wang et al.210 They utilized macroporous n-type GaP and poly(3,4-ethylenedioxythiophene):poly(4-styrene sulfonate) (PEDOT:PSS) to synthesize such solar cells. The high aspect ratio structure of macroporous GaP led to higher photocurrents as well as an external quantum yield according to the wavelength.
Hybrid macroporous GaP/PEDOT:PSS devices displayed a JSC of 2.34 mA cm−2, a VOC of 0.95 V, a FF of 0.54, and an overall efficiency of 1.21% under 1.0 sun illumination. By using current density–voltage measurements, the extent of the effect of the dopant density of GaP on the performance of the hybrid devices can be probed. Gold-coated macroporous GaP, prior to PEDOT:PSS coating, exhibited increased device performance, with an efficiency of 1.81%. On the contrary, planar GaP/PEDOT:PSS modified with gold displayed a lower external quantum yield over all wavelengths and reduced JSC and VOC values.
4.4. Zinc phosphide materials
Graphene has become an attractive material for optoelectronic and photovoltaic devices, with extraordinary properties such as high carrier mobility and high optical transparency.211,212 The barrier tuning of a graphene–Zn3P2 field-effect solar cell with a semitransparent top-gate electrode was studied by Oscar et al. and they demonstrated that this tuning could embellish the photovoltaic efficiency of solar cells.213Adding a semitransparent top electrostatic gate allows for the tuning of the graphene Fermi level, and as a consequence, the energy barrier at the graphene–Zn3P2 joint goes from an ohmic contact at negative gate voltages to a rectifying barrier at positive gate voltages. As photovoltaic measurements show, the efficiency conversion increased 2-fold when they increased the junction barrier and gate voltages to maximize the photovoltaic response. The photovoltaic current and voltage are shown in Fig. 28. An open-circuit voltage VOC = 0.53 V, as well as an efficiency of 1.9% under AM 1.5 1 sun solar illumination, were obtained at a first-rank gate voltage of +2 V.
 | ||
| Fig. 28 The photovoltaic response of a cell under AM 1.5 illumination showing the photocurrent (top) and power (bottom) as a function of VB for different gate voltages. Adapted with permission from ref. 213, © 2014 American Chemical Society. | ||
5. Electrocatalysis
5.1 Hydrogen evolution reaction
 | ||
| Fig. 29 (a) TEM image of Co2P nanorods; (b) SEM image of CoP NPAs; (c) TEM image of CoP nanoparticles; (d) low-magnification SEM image of as-prepared CoP NCs. (a) Adapted with permission from ref. 214, © 2014 Elsevier; (b) ref. 215, © 2015 the Royal Society of Chemistry; (c) ref. 216, © 2014 Wiley; (d) ref. 217, © 2015 American Chemical Society. | ||
 | ||
| Fig. 30 (a) TEM image of Ni2P nanoparticles; (b) high-magnification SEM image of a Ni5P4–Ni2P-NS array cathode; (c) FE-SEM image of MoP; (d) high-magnification SEM image of Cu3P NW/CF. (a) Adapted with permission from ref. 219, © 2013 American Chemical Society; (b) ref. 220, © 2015 Wiley; (c) ref. 223, © 2014 the Royal Society of Chemistry; (d) ref. 224, © 2014 Wiley. | ||
 | ||
| Fig. 31 (a) The morphology of Ni5P4 after 500 cycles through SEM imaging; (b) SEM image of CoP2/RGO; (c) TEM image of BNC/Co2P-2; (d) SEM image of CoP; (a) adapted with permission from ref. 13, © 2015 Wiley; (b) ref. 226, © 2016 the Royal Society of Chemistry; (c) ref. 231, © 2015 the Royal Society of Chemistry; (d) ref. 59, © 2015 Elsevier. | ||
Li et al. demonstrated that various nanostructured cobalt phosphide assemblies can be synthesized through the direct chemical transformation of a Co-containing metal–organic framework (ZIF-67-Co) under mild phosphorization conditions.215 Furthermore, Fig. 29b shows a typical low-magnification SEM image of CoP nanoparticle assemblies (NPAs). And this suggests that the as-prepared CoP is composed of a large number of irregular polyhedron-like particles. In addition, the resulting CoP nanoparticle assemblies (NPAs) and nanorod assemblies (NRAs) can be selectively synthesized by rationally tuning the calcination atmosphere. And for the HER, CoP NRAs were more highly active than CoP NPAs, which can be proved by their relatively low onset overpotential (similar to 86 mV), small Tafel slope (similar to 69 mV per decade) and long-term stability in acidic media.
Popczun et al. successfully synthesized cobalt phosphide nanoparticles via the reaction between Co nanoparticles and trioctylphosphine.216 And then the as-obtained nanoparticles were utilized as electrocatalysts for the HER under strongly acidic conditions (0.50 M H2SO4, pH 0.3). Fig. 29c displays a TEM image of the CoP nanoparticles. In addition, electrodes based on CoP nanoparticles supported by Ti (2 mg cm−2 mass loading) produced a cathodic current density of 20 mA cm−2 at an overpotential of −85 mV. Furthermore, the CoP/Ti electrodes could be stable over 24 h for the HER in 0.50 M H2SO4. To be specific, the activity can remain unchanged after 400 cyclic voltammetry sweeps, which indicates long-term viability under the operating conditions.
Yang et al. fabricated urchin-like CoP nanocrystals (NCs) and these nanocrystals can be utilized as catalysts for the HER.217 The as-prepared CoP NCs held excellent electrocatalytic activity and long-term stability. A low-magnification SEM image of the as-prepared CoP NCs is displayed in Fig. 29d. In addition, the urchin-like CoP NCs, with a diameter of 5 μm, could be synthesized via a hydrothermal reaction following phosphidation treatment under an N2 atmosphere. CoP NCs deliver an excellent HER catalytic performance, with a low onset overpotential of 50 mV, a small Tafel slope of 46 mV per decade, and an exceptional low overpotential of similar to 180 mV at a current density of 100 mA cm−2 with a mass loading density of 0.28 mg cm−2. More importantly, the urchin-like CoP NCs present superior stability and keep their catalytic activity for at least 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CV cycles for the HER in 0.5 M H2SO4, which is attributed to their three-dimensional structure.
000 CV cycles for the HER in 0.5 M H2SO4, which is attributed to their three-dimensional structure.
Ni2P nanoparticles have been investigated by Popczun et al. for the HER in acidic solutions, under which proton exchange membrane-based electrolysis is operational.219 A TEM image of the Ni2P nanoparticles is displayed in Fig. 30a. What is more, the Ni2P nanoparticles which were catalytically active were hollow and faceted to expose a high density of the Ni2P (001) surface. And these have been proved to be active HER catalysts.
Wang et al. presented an approach for the synthesis of a three-dimensional self-supported biphasic Ni5P4–Ni2P nanosheet (NS) array cathode.220 The target can be obtained through the direct phosphorization of commercially available nickel foam using phosphorus vapor. Fig. 30b shows a high-magnification SEM image of the Ni5P4–Ni2P-NS array cathode. What is more, the as-prepared three-dimensional Ni5P4–Ni2P-NS array cathode delivers excellent electrocatalytic activity and long-term durability for the HER in an acidic medium. In addition, the fabrication procedure is scalable, which predicts the prospect for utilization in water electrolysis and other self-supported TMNP HER cathodes.
Liang et al. prepared self-supported FeP nanorod arrays on carbon cloth (FeP NAs/CC) via the low-temperature phosphidation of Fe2O3 NAs/CC.37 As a novel 3D hydrogen evolution cathode in acidic media, FeP NAs/CC delivers high catalytic activity. What is more, FeP NAs/CC only needs an overpotential of 58 mV to afford a current density of 10 mA cm−2. This electrode also works efficiently in both neutral and alkaline solutions.
Xiao et al. proposed that molybdenum phosphide was a novel cost-effective catalyst (a FE-SEM image of MoP is shown in Fig. 30c), which displays high activity for the HER in both acid and alkaline media, even in bulk form.223 Comparative analysis of Mo, Mo3P and MoP as catalysts towards the HER manifests that the phosphorization process can modify the properties of the metal, and in addition, different degrees of phosphorization lead to distinct activities and stabilities. What is more, theoretical calculations via density functional theory also indicate the simple phosphorization of molybdenum to synthesize MoP. And the theoretical calculations introduce an excellent H-delivery system which attains nearly zero binding to H at a certain H coverage.
Tian et al. proposed that self-supported Cu3P nanowire arrays grown on commercial porous copper foam (Cu3P NW/CF) could be synthesized via a low-temperature phosphidation reaction.224 Fig. 30d displays a high-magnification SEM image of Cu3P NW/CF. The reaction begins from a Cu(OH)2 NW/CF precursor. Furthermore, when used as a three-dimensional HER cathode in acidic electrolyte, Cu3P NW/CF can maintain its activity for at least 25 hours and deliver an onset overpotential of 62 mV, a Tafel slope of 67 mV per decade, and a faradaic efficiency close to 100%. The catalytic current density can approach 10 mA cm−2 at an overpotential of 143 mV.
5.2 Photoelectrochemical water splitting
Wu et al. fabricated nanocrystalline nickel phosphide (Ni12P5) through a simple hydrothermal method, using NiCl2 and red phosphorus as raw materials.225 And then they characterized the crystal structure, morphology and surface chemical composition of the as-prepared sample via XRD, SEM and XPS, respectively. The catalytic activity for the HER from water was investigated under visible light irradiation with fluorescein sodium as the photosensitizer and triethanolamine as the sacrificial electron donor. And the Ni12P5 sample showed high catalytic activity (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 mmol h−1 g−1, TOF = 9.3 h−1) and good stability (15 h). The results indicated that Ni12P5 held a high cathodic current and small charge transfer resistance, which further proved that Ni12P5 could efficiently catalyze the HER.
760 mmol h−1 g−1, TOF = 9.3 h−1) and good stability (15 h). The results indicated that Ni12P5 held a high cathodic current and small charge transfer resistance, which further proved that Ni12P5 could efficiently catalyze the HER.
Jin et al. prepared uniform Co2P ultrathin nanowires via a facile and rapid microwave-assisted method.227 And Co2P can be utilized as a catalyst for the water splitting reaction, and the results show that the as-prepared samples are excellent bifunctional electrocatalysts. In acidic and alkaline electrolyte, both Co2P and CoP NWs show a low overpotential and small Tafel slope for the HER, close to those of platinum. In addition, Co2P nanowires display the best performance among CoP nanowires and RuO2 towards the OER. According to mechanistic studies, cobalt oxo/hydroxide with phosphate units are formed in situ during the catalysis of water oxidation. Therefore, an alkaline water electrolysis cell was constructed based on the Co2P NWs as both the anode and cathode. The electricity-to-hydrogen conversion efficiency was ∼84%, which is higher than for Pt/RuO2.
Semimetallic MoP2 nanoparticles were found to be a novel photocatalyst to efficiently degenerate methyl orange and produce H2 from water under visible light irradiation.230 And MoP2 nanoparticles were synthesized by utilizing a solid-state reaction route via a vacuum encapsulation technique, followed by acid washing. Both first-principle band-structure calculations and experimental measurements revealed typical semimetallic characteristics for MoP2. The obtained MoP2 nanoparticles displayed superior photocatalytic performance for the degradation of methyl orange with good stability and the reduction of water assisted by the sacrificial elemental Pt under visible light. The detection of hydroxyl radicals in solution in the presence of MoP2 with fluorescence spectroscopy confirmed the photodegradable activity.
5.3 Oxygen reduction reaction
Dicobalt phosphide nanoparticles encased in boron and nitrogen co-doped graphitic layers (BNC/Co2P) were fabricated by Han et al.231 A TEM image of BNC/Co2P-2 is shown in Fig. 31c. The Co2P nanoparticle core played a positive role in activating the B, N co-doped graphitic shell towards the ORR. And they utilized a one-step pyrolysis method, and BNC/Co2P exhibited excellent electrocatalytic oxygen reduction reaction (ORR) performance compared with commercial Pt/C towards the ORR via a four-electron pathway in alkaline solution, with long-term stability and excellent methanol tolerance.Carbon supported PtP (PtP/C) catalysts were synthesized by Ma et al. from Pt(NO3)2 and phosphorus yellow at room temperature.232 And the content of P in the as-prepared PtP/C catalyst was high, in addition, as the content of P increased, the average size of the PtP particles decreased. The electrocatalytic performance of the PtP/C catalysts prepared with this method for the ORR was better than that of a commercial Pt/C catalyst. And the reason why P can promote the enhancement in the electrocatalytic performance of the PtP/C catalyst for the ORR is that Pt and P can form an alloy and then the electron density of Pt is decreased.
5.4 Other electrocatalysis
A carbon nanotube (CNT) supported Pt–cobalt phosphide (CoP) electrocatalyst (Pt/CoP/CNTs) was designed and prepared by Li et al. for methanol oxidation (MOR).234 The modification of CoP decreases the Pt particle size significantly and increases the electrochemical surface area due to the interaction between Pt and CoP, which is evidenced by transmission electron microscopy, X-ray diffraction and X-ray photoelectron spectroscopy. Among all these catalysts, the Pt/4% CoP/CNTs catalyst exhibits the best MOR activity of 1600 mA mg−1 Pt, which is six times that of Pt/CNTs. Moreover, this catalyst also exhibits a higher onset current density and more steady current density than other Pt-based catalysts. The work provides a promising method to develop highly active and stable Pt-based catalysts for direct methanol fuel cells.
Through a productive and simple ball-milling method, Li et al. synthesized CoP. A SEM image of the as-prepared sample is displayed in Fig. 31d. And then it was tested as an anode material for NIBs. Furthermore, the as-obtained CoP can exhibit a large initial capacity of 770 mA h g−1 and deliver lower voltage polarization. Via ex situ XPS and STEM characterization, it was concluded that the sodium storage mechanism of CoP involves a reaction of P with Na.
6. Conclusion and outlook
In summary, we have summarized the synthetic strategies that are suitable for TMPNs and stated several applications to electrochemical storage and conversion, including Li-ion batteries, Na-ion batteries, supercapacitors, solar cells and electrocatalysis. In recent years, TMPNs have been proven to be one of the most promising and excellent materials in the field of electrochemistry. Several points in regard to the developing trends in TMPNs in electrochemical energy storage and conversion are listed as follows:(1) Although a good deal of effort has been made and great advancement has been performed in Li-ion batteries and Na-ion batteries recently, there are still some problems that require solving. What should be further established are synthetic methods, experimental methods and relevant theory, coupled with the exploration of new and applied materials.236 And Na-ion batteries are both similar and different from Li-ion batteries, and therefore, research based on Na-ion batteries is faced with the same challenges as for Li-ion batteries, for example, capacity fading on cycling, and electrolyte decomposition when there is a high voltage. The exploration of new materials for Na-ion batteries and Li-ion batteries is imperative, including solvents, active electrode materials, carbon additives, binders, electrolyte salts, and so on.
(2) As for supercapacitors, limited energy density is the primary challenge impeding their extensive energy storage application. And it is the discovery of new electrode materials that can help us surmount this primary challenge. Especially, the fabrication of composite materials is a significant strategy for boosting the performance of supercapacitors. Wang et al. put forward four favored properties of supercapacitor electrode materials: (i) a high specific surface area, (ii) suitable pore-size distribution, pore networks, and pore length, (iii) low internal electrical resistance, and (iv) better cycling stability.237
(3) The main challenge in DSSCs is to overcome the loss of potential drop in the process of regeneration, and hence, trying to construct a system in which the regeneration of the oxidized dye can come true is necessary. It is a fact that components with superior performance have been developed. When combined with other substances forming composites, TMPNs could turn into excellent materials, and this is a promising field worth exploring.
Abbreviation index
| TMPNs | Transition metal phosphide nanomaterials |
| h-Ni2P | Hierarchical nanostructured nickel phosphide |
| TOP | Trioctylphosphine |
| OAm | Oleylamine |
| TF-VLS | Thin-film vapor–liquid–solid |
| rGO | Reduced graphene oxide |
| LIBs | Li-ion batteries |
| HEVs | Hybrid electric vehicles |
| EVs | Electric vehicles |
| EIS | Electrochemical impedance spectrum |
| SEM | Scanning electronic microscopy |
| FEGSEM | Field emission gun scanning electronic microscopy |
| TEM | Transmission electron microscopy |
| BET | Brunauer–Emmett–Teller |
| OA | Oleic acid |
| THF | Tetrahydrofuran |
| XRD | X-ray diffraction |
| CV | Cyclic voltammetry |
| SAED | Selected-area electron diffraction |
| NIBs | Na-ion batteries |
| FEC | Fluoroethylene carbonate |
| SEI | Stable solid-electrolyte interphase |
| HRTEM | High resolution transmission electron microscopy |
| CMC | Carboxymethyl cellulose |
| CMC/CB | Carboxymethyl cellulose/carbon black |
| SCs | Supercapacitors |
| EDLCs | Electrical double-layer capacitors |
| PDCs | Pseudocapacitors |
| Ni2P NS/NF | Ni foam-supported Ni2P nanosheet |
| NPs | Nanoparticles |
| GR | Graphene |
| Ni2P//AC ASCs | Ni2P nanoparticles and activated carbon |
| DSSCs | Dye-sensitized solar cells |
| PV | Photovoltaic |
| PEMFCs | Proton exchange membrane fuel cells |
| PR | Pulse-reverse |
| PS | Potentiostatic |
| FTO | Fluorine-doped tin oxide |
| PCE | Power conversion efficiency |
| J–V | Photocurrent–voltage |
| CE | Counter electrode |
| MC | Mesoporous carbon: |
| VOC | Open-circuit voltage |
| FF | Fill factor |
| JSC | Short-circuit current |
| MOVPE | Metal-organic vapor phase epitaxy |
| HER | Hydrogen evolution reaction |
| ORR | Oxygen reduction reaction |
| NCs | Nanocrystals |
| XAFS | X-ray absorption fine structure |
| OER | Oxygen evolution reaction |
| MOR | Methanol oxidation |
| NPAs | Nanoparticle assemblies |
| NRAs | Nanorod assemblies |
| NCs | Nanocrystals |
| RHE | Reversible hydrogen electrode |
| DFT | Density functional theory |
| NS | Nanosheet |
| PEC | Photoelectrochemical |
| RHE | Reversible hydrogen electrode |
| CNTs | Carbon nanotubes |
Acknowledgements
This work was supported by the Program for New Century Excellent Talents of the University in China (grant no. NCET-13-0645) and the National Natural Science Foundation of China (NSFC-21201010,21671170 21173183, 21505118 and 51202106), Innovation Scientists and Technicians Troop Construction Projects of Henan Province (164200510018), the Plan for Scientific Innovation Talent of Henan Province, the Program for Innovative Research Team (in Science and Technology) in the University of Henan Province (14IRTSTHN004, 16IRTSTHN003), the Science & Technology Foundation of Henan Province (122102210253 and 13A150019), the Science & Technology Foundation of Jiangsu Province (BK20150438), the Six Talent Plan (2015-XCL-030), Innovation and Entrepreneurship Training Program for College Students in Jiangsu province (No. 201611117047Y), and the China Postdoctoral Science Foundation (2012M521115). We also acknowledge the Priority Academic Program Development of Jiangsu Higher Education Institutions and the technical support we received at the Testing Center of Yangzhou University.Notes and references
- C. Deng, F. Ding, X. Li, Y. Guo, W. Ni, H. Yan, K. Sun and Y. Yan, J. Mater. Chem. A, 2016, 4, 59–66 CAS.
- C. Lv, Z. Chen, Z. Chen, B. Zhang, Y. Qin, Z. Huang and C. Zhang, J. Mater. Chem. A, 2015, 3, 17669–17675 CAS.
- J. Li, X. Zhou, Z. Xia, Z. Zhang, J. Li, Y. Ma and Y. Qu, J. Mater. Chem. A, 2015, 3, 13066–13071 CAS.
- Y. Pan, W. Hu, D. Liu, Y. Liu and C. Liu, J. Mater. Chem. A, 2015, 3, 13087–13094 CAS.
- B. Phuong, J. A. Cecilia, S. T. Oyama, A. Takagaki, A. Infantes-Molina, H. Zhao, D. Li, E. Rodriguez-Castellon and A. Jimenez Lopez, J. Catal., 2012, 294, 184–198 CrossRef.
- Y. Shi and B. Zhang, Chem. Soc. Rev., 2016, 45, 1529–1541 RSC.
- M. Sun, H. Liu, J. Qu and J. Li, Adv. Energy Mater., 2016, 6 DOI:10.1002/aenm.201600087.
- J. Tian, N. Cheng, Q. Liu, X. Sun, Y. He and A. M. Asiri, J. Mater. Chem. A, 2015, 3, 20056–20059 CAS.
- H. Zhu, J. Zhang, R. Yanzhang, M. Du, Q. Wang, G. Gao, J. Wu, G. Wu, M. Zhang, B. Liu, J. Yao and X. Zhang, Adv. Mater., 2015, 27, 4752–4759 CrossRef CAS PubMed.
- H. Du, Q. Liu, N. Cheng, A. M. Asiri, X. Sun and C. M. Li, J. Mater. Chem. A, 2014, 2, 14812–14816 CAS.
- P. Jiang, Q. Liu, C. Ge, W. Cui, Z. Pu, A. M. Asiri and X. Sun, J. Mater. Chem. A, 2014, 2, 14634–14640 CAS.
- M. Xu, L. Han, Y. Han, Y. Yu, J. Zhai and S. Dong, J. Mater. Chem. A, 2015, 3, 21471–21477 CAS.
- M. Ledendecker, S. K. Calderon, C. Papp, H.-P. Steinrueck, M. Antonietti and M. Shalom, Angew. Chem., Int. Ed., 2015, 54, 12361–12365 CrossRef CAS PubMed.
- R. S. Devan, R. A. Patil, J.-H. Lin and Y.-R. Ma, Adv. Funct. Mater., 2012, 22, 3326–3370 CrossRef CAS.
- S. Faraji and F. N. Ani, J. Power Sources, 2014, 263, 338–360 CrossRef CAS.
- L. Ji, Z. Lin, M. Alcoutlabi and X. Zhang, Energy Environ. Sci., 2011, 4, 2682–2699 CAS.
- F. Wang, Z. a. Tan and Y. Li, Energy Environ. Sci., 2015, 8, 1059–1091 CAS.
- W. Wei, Z. Wang, Z. Liu, Y. Liu, L. He, D. Chen, A. Umar, L. Guo and J. Li, J. Power Sources, 2013, 238, 376–387 CrossRef CAS.
- Z.-S. Wu, G. Zhou, L.-C. Yin, W. Ren, F. Li and H.-M. Cheng, Nano Energy, 2012, 1, 107–131 CrossRef CAS.
- Y. Ren, Z. Ma and P. G. Bruce, Chem. Soc. Rev., 2012, 41, 4909–4927 RSC.
- K. Zhang, X. Han, Z. Hu, X. Zhang, Z. Tao and J. Chen, Chem. Soc. Rev., 2015, 44, 699–728 RSC.
- Z. Wang, J. S. Chen, T. Zhu, S. Madhavi and X. W. Lou, Chem. Commun., 2010, 46, 6906–6908 RSC.
- M.-S. Balogun, W. Qiu, W. Wang, P. Fang, X. Lu and Y. Tong, J. Mater. Chem. A, 2015, 3, 1364–1387 CAS.
- R. Chen, X. Tu and D. Chen, Prog. Chem., 2015, 27, 416–423 Search PubMed.
- S. Dong, X. Chen, X. Zhang and G. Cui, Coord. Chem. Rev., 2013, 257, 1946–1956 CrossRef CAS.
- C. Zhang, H. B. Wu, Z. Guo and X. W. Lou, Electrochem. Commun., 2012, 20, 7–10 CrossRef CAS.
- M. Sun, H. Liu, J. Qu and J. Li, Adv. Energy Mater., 2016, 6, 1–34 Search PubMed.
- G. Shi and J. Shen, J. Mater. Chem., 2009, 19, 2295–2297 RSC.
- S. Carenco, D. Portehault, C. Boissiere, N. Mezailles and C. Sanchez, Adv. Mater., 2014, 26, 371–389 CrossRef CAS PubMed.
- X. Huang, C. Tan, Z. Yin and H. Zhang, Adv. Mater., 2014, 26, 2185–2204 CrossRef CAS PubMed.
- J. Lu, C. Nan, Q. Peng and Y. Li, J. Power Sources, 2012, 202, 246–252 CrossRef CAS.
- A. E. Henkes, Y. Vasquez and R. E. Schaak, J. Am. Chem. Soc., 2007, 129, 1896–1897 CrossRef CAS PubMed.
- W. Cui, Q. Liu, N. Cheng, A. M. Asiri and X. Sun, Chem. Commun., 2014, 50, 9340–9342 RSC.
- W. Cui, Q. Liu, Z. Xing, A. M. Asiri, K. A. Alamry and X. Sun, Appl. Catal., B, 2015, 164, 144–150 CrossRef CAS.
- P. Jiang, Q. Liu, Y. Liang, J. Tian, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2014, 53, 12855–12859 CrossRef CAS PubMed.
- P. Jiang, Q. Liu and X. Sun, Nanoscale, 2014, 6, 13440–13445 RSC.
- Y. Liang, Q. Liu, A. M. Asiri, X. Sun and Y. Luo, ACS Catal., 2014, 4, 4065–4069 CrossRef CAS.
- Q. Liu, Z. Pu, A. M. Asiri and X. Sun, Electrochim. Acta, 2014, 149, 324–329 CrossRef CAS.
- Q. Liu, J. Tian, W. Cui, P. Jiang, N. Cheng, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2014, 53, 6710–6714 CrossRef CAS PubMed.
- Z. Xing, Q. Liu, A. M. Asiri and X. Sun, Adv. Mater., 2014, 26, 5702–5707 CrossRef CAS PubMed.
- Z. Xing, Q. Liu, A. M. Asiri and X. Sun, ACS Catal., 2015, 5, 145–149 CrossRef CAS.
- J. Wang, W. Cui, Q. Liu, Z. Xing, A. M. Asiri and X. Sun, Adv. Mater., 2016, 28, 215–230 CrossRef CAS PubMed.
- E. Muthuswamy and S. L. Brock, J. Am. Chem. Soc., 2010, 132, 15849–15851 CrossRef CAS PubMed.
- C. Qian, F. Kim, L. Ma, F. Tsui, P. D. Yang and J. Liu, J. Am. Chem. Soc., 2004, 126, 1195–1198 CrossRef CAS PubMed.
- H. Song, M. Dai, H.-L. Song, X. Wan, X.-W. Xu and Z.-S. Jin, J. Mol. Catal. A: Chem., 2014, 385, 149–159 CrossRef CAS.
- S. Boyanov, K. Annou, C. Villevieille, M. Pelosi, D. Zitoun and L. Monconduit, Ionics, 2008, 14, 183–190 CrossRef CAS.
- W. Peng, L. Jiao, Q. Huan, L. Li, J. Yang, Q. Zhao, Q. Wang, H. Du, G. Liu, Y. Si, Y. Wang and H. Yuan, J. Alloys Compd., 2012, 511, 198–201 CrossRef CAS.
- Y. Lu, J.-P. Tu, Q.-Q. Xiong, J.-Y. Xiang, Y.-J. Mai, J. Zhang, Y.-Q. Qiao, X.-L. Wang, C.-D. Gu and S. X. Mao, Adv. Funct. Mater., 2012, 22, 3927–3935 CrossRef CAS.
- G. Shi and J. Shen, Catal. Commun., 2009, 10, 1693–1696 CrossRef CAS.
- H. Pfeiffer, F. Tancret and T. Brousse, Electrochim. Acta, 2005, 50, 4763–4770 CrossRef CAS.
- T. X. Wang, H. Xiao and Y. C. Zhang, Mater. Lett., 2008, 62, 3736–3738 CrossRef CAS.
- R. Prins and M. E. Bussell, Catal. Lett., 2012, 142, 1413–1436 CrossRef CAS.
- W. Tebib, S. Alleg, R. Bensalem, N. Bensebaa, F. Z. Bentayeb, J. J. Sunol and J. M. Greneche, J. Nanosci. Nanotechnol., 2008, 8, 2029–2036 CrossRef CAS PubMed.
- S. Brutti, G. Mulas, E. Piciollo, S. Panero and P. Reale, J. Mater. Chem., 2012, 22, 14531–14537 RSC.
- W. Li, H. Li, Z. Lu, L. Gan, L. Ke, T. Zhai and H. Zhou, Energy Environ. Sci., 2015, 8, 3629–3636 CAS.
- M. C. Stan, R. Kloepsch, A. Bhaskar, J. Li, S. Passerini and M. Winter, Adv. Energy Mater., 2013, 3, 231–238 CrossRef CAS.
- Y. Kim, Y. Kim, A. Choi, S. Woo, D. Mok, N. S. Choi, Y. S. Jung, J. H. Ryu, S. M. Oh and K. T. Lee, Adv. Mater., 2014, 26, 4139–4144 CrossRef CAS PubMed.
- W. Li, S. L. Chou, J. Z. Wang, J. H. Kim, H. K. Liu and S. X. Dou, Adv. Mater., 2014, 26, 4037–4042 CrossRef CAS PubMed.
- W.-J. Li, Q.-R. Yang, S.-L. Chou, J.-Z. Wang and H.-K. Liu, J. Power Sources, 2015, 294, 627–632 CrossRef CAS.
- M. S. Chandrasekar and S. Mitra, Ionics, 2014, 20, 137–140 CrossRef CAS.
- Y. Lu, C. D. Gu, X. Ge, H. Zhang, S. Huang, X. Y. Zhao, X. L. Wang, J. P. Tu and S. X. Mao, Electrochim. Acta, 2013, 112, 212–220 CrossRef CAS.
- J. Y. Xiang, X. L. Wang, J. Zhong, D. Zhang and J. P. Tu, J. Power Sources, 2011, 196, 379–385 CrossRef CAS.
- J. Liu, P. Kopold, C. Wu, P. A. van Aken, J. Maier and Y. Yu, Energy Environ. Sci., 2015, 8, 3531–3538 CAS.
- Y. Lu, J. P. Tu, J. Y. Xiang, X. L. Wang, J. Zhang, Y. J. Mai and S. X. Mao, J. Phys. Chem. C, 2011, 115, 23760–23767 CAS.
- Y. Bai, H. Zhang, X. Li, L. Liu, H. Xu, H. Qiu and Y. Wang, Nanoscale, 2015, 7, 1446–1453 RSC.
- J. W. Hall, N. Membreno, J. Wu, H. Celio, R. A. Jones and K. J. Stevenson, J. Am. Chem. Soc., 2012, 134, 5532–5535 CrossRef CAS PubMed.
- K. Yan, Y. Li, X. Zhang, X. Yang, N. Zhang, J. Zheng, B. Chen and K. J. Smith, Int. J. Hydrogen Energy, 2015, 40, 16137–16146 CrossRef CAS.
- Y. Chen, H. She, X. Luo, G.-H. Yue and D.-L. Peng, J. Cryst. Growth, 2009, 311, 1229–1233 CrossRef CAS.
- H. Li, W. Wang, Z. Gong, Y. Yu, L. Piao, H. Chen and J. Xia, J. Phys. Chem. Solids, 2015, 80, 22–25 CrossRef CAS.
- T. H. Guo, Y. Liu, Y. C. Zhang and M. Zhang, Mater. Lett., 2011, 65, 639–641 CrossRef CAS.
- Q. L. Huang, H. Chen, Y. C. Zhang and C. L. Wu, J. Alloys. Compd., 2011, 509, 6382–6387 CrossRef CAS.
- Y. C. Zhang, G. Y. Wang, X. Y. Hu and W. W. Chen, Mater. Res. Bull., 2006, 41, 1817–1824 CrossRef CAS.
- W. Zhang, B. Quan, C. Lee, S. K. Park, X. Li, E. Choi, G. W. Diao and Y. Piao, ACS Appl. Mater. Interfaces, 2015, 7, 2404–2414 CAS.
- Y. Y. Dou, G. R. Li, J. Song and X. P. Gao, Phys. Chem. Chem. Phys., 2012, 14, 1339–1342 RSC.
- S. Liu, H. Zhang, L. Xu, L. Ma and X. Chen, J. Power Sources, 2016, 304, 346–353 CrossRef CAS.
- W. Du, S. Wei, K. Zhou, J. Guo, H. Pang and X. Qian, Mater. Res. Bull., 2015, 61, 333–339 CrossRef CAS.
- K. Aso, A. Hayashi and M. Tatsumisago, Inorg. Chem., 2011, 50, 10820–10824 CrossRef CAS PubMed.
- Y. Lu, J.-P. Tu, Q.-Q. Xiong, Y.-Q. Qiao, X.-L. Wang, C.-D. Gu and S. X. Mao, RSC Adv., 2012, 2, 3430–3436 RSC.
- C. An, Y. Wang, Y. Wang, G. Liu, L. Li, F. Qiu, Y. Xu, L. Jiao and H. Yuan, RSC Adv., 2013, 3, 4628–4633 RSC.
- M. Zheng, H.-P. Wang, C. M. Sutter-Fella, C. Battaglia, S. Aloni, X. Wang, J. Moore, J. W. Beeman, M. Hettick, M. Amani, W.-T. Hsu, J. W. Ager, P. Bermel, M. Lundstrom, J.-H. He and A. Javey, Adv. Energy Mater., 2015, 5, 201501337 Search PubMed.
- A. Guerfi, M. Dontigny, P. Charest, M. Petitclerc, M. Lagace, A. Vijh and K. Zaghib, J. Power Sources, 2010, 195, 845–852 CrossRef CAS.
- D. McNulty, D. N. Buckley and C. O'Dwyer, J. Power Sources, 2014, 267, 831–873 CrossRef CAS.
- Y. Wu, Z. Wen and J. Li, Adv. Mater., 2011, 23, 1126–1129 CrossRef CAS PubMed.
- S. Afyon, D. Kundu, F. Krumeich and R. Nesper, J. Power Sources, 2013, 224, 145–151 CrossRef CAS.
- L. Bodenes, A. Darwiche, L. Monconduit and H. Martinez, J. Power Sources, 2015, 273, 14–24 CrossRef CAS.
- J. Chen, L. Yang, S. Fang and Y. Tang, Electrochim. Acta, 2010, 55, 6596–6600 CrossRef CAS.
- S. Goriparti, E. Miele, F. De Angelis, E. D. Fabrizio, R. P. Zaccaria and C. Capiglia, J. Power Sources, 2014, 257, 421–443 CrossRef CAS.
- J. Liu and Q. Pan, Electrochem. Solid-State Lett., 2010, 13, 404–412 Search PubMed.
- F.-M. Courtel, Y. Abu-Lebdeh and I.-J. Davidson, Electrochim. Acta, 2012, 71, 123–127 CrossRef CAS.
- X.-M. Liu, Z.-D. Huang, S. Oh, P.-C. Ma, P. C. H. Chan, G. K. Vedam, K. Kang and J.-K. Kim, J. Power Sources, 2010, 195, 4290–4296 CrossRef CAS.
- B. Zhang, Z.-D. Huang, S. W. Oh and J.-K. Kim, J. Power Sources, 2011, 196, 10692–10697 CrossRef CAS.
- H. Zheng, J. Li, X. Song, G. Liu and V.-S. Battaglia, Electrochim. Acta, 2012, 71, 258–265 CrossRef CAS.
- P. Hovington, M. Dontigny, A. Guerfi, J. Trottier, M. Lagace, A. Mauger, C. M. Julien and K. Zaghib, J. Power Sources, 2014, 248, 457–464 CrossRef CAS.
- X. Liu, J. Liu, T. Huang and A. Yu, Electrochim. Acta, 2013, 109, 52–58 CrossRef CAS.
- Q. Kuang, Y. Zhao and Z. Liang, J. Power Sources, 2011, 196, 10169–10175 CrossRef CAS.
- Z. Liang, Z. Lin, Y. Zhao, Y. Dong, Q. Kuang, X. Lin, X. Liu and D. Yan, J. Power Sources, 2015, 274, 345–354 CrossRef CAS.
- J. Liu, Y. Zhou, J. Wang, Y. Pan and D. Xue, Chem. Commun., 2011, 47, 10380–10382 RSC.
- S. Ni, X. Lv, J. Ma, X. Yang and L. Zhang, J. Power Sources, 2014, 248, 122–129 CrossRef CAS.
- G. Li, X. Feng, Y. Ding, S. Ye and X. Gao, Electrochim. Acta, 2012, 78, 308–315 CrossRef CAS.
- Y. Wu, Z. Wen and J. Li, Adv. Mater., 2011, 23, 1126–1129 CrossRef CAS PubMed.
- Z. Wang, E. Liu, C. He, C. Shi, J. Li and N. Zhao, J. Power Sources, 2013, 236, 25–32 CrossRef CAS.
- T. Yi, S. Yang and Y. Xie, J. Mater. Chem., 2015, 3, 5750–5777 RSC.
- K. Zaghib, M. Dontigny, A. Guerfi, P. Charest, I. Rodrigues, A. Mauger and C. M. Julien, J. Power Sources, 2011, 196, 3949–3954 CrossRef CAS.
- S. Liu, J. Feng, X. Bian, J. Liu and H. Xu, RSC Adv., 2015, 5, 60870–60875 RSC.
- H. Zhang, Y. Feng, Y. Zhang, L. Fang, W. Li, Q. Liu, K. Wu and Y. Wang, ChemSusChem, 2014, 7, 2000–2006 CrossRef CAS PubMed.
- Y. Lu, J.-P. Tu, Q.-Q. Xiong, H. Zhang, C.-D. Gu, X.-l. Wang and S. X. Mao, CrystEngComm, 2012, 14, 8633–8641 RSC.
- Y.-H. Cui, M.-Z. Xue, Z.-W. Fu, X.-L. Wang and X.-J. Liu, J. Alloys. Compd., 2013, 555, 283–290 CrossRef CAS.
- S. Ni, J. Ma, X. Lv, X. Yang and L. Zhang, J. Mater. Chem. A, 2014, 2, 20506–20509 CAS.
- M. Mortazavi, J. Deng, V. B. Shenoy and N. V. Medhekar, J. Power Sources, 2013, 225, 207–214 CrossRef CAS.
- J. Cabana, L. Monconduit, D. Larcher and M. R. Palacin, Adv. Mater., 2010, 22, E170–E192 CrossRef CAS PubMed.
- H. Bian, J. Zhang, M.-F. Yuen, W. Kang, Y. Zhan, D. Y. W. Yu, Z. Xu and Y. Y. Li, J. Power Sources, 2016, 307, 634–640 CrossRef CAS.
- S.-Y. Hong, Y. Kim, Y. Park, A. Choi, N.-S. Choic and K.-T. Lee, Energy Environ. Sci., 2013, 6, 2067–2081 CAS.
- S. Mukherjee, A. Bates, N. Schuppert, B. Son, J. G. Kim, J. S. Choi, M. J. Choi, D.-H. Lee, O. Kwon, J. Jasinski and S. Park, J. Power Sources, 2015, 286, 276–289 CrossRef CAS.
- A. Darwiche, M.-T. Sougrati, B. Fraisse, L. Stievano and L. Monconduit, Electrochem. Commun., 2013, 32, 18–21 CrossRef CAS.
- P. R. Kumar, Y. H. Jung, K. K. Bharathi, C. H. Lim and D. K. Kim, Electrochim. Acta, 2014, 146, 503–510 CrossRef CAS.
- L. Baggetto, H.-Y. Hah, J.-C. Jumas, C. E. Johnson, J. A. Johnson, J. K. Keum, C. A. Bridges and G. M. Veith, J. Power Sources, 2014, 267, 329–336 CrossRef CAS.
- M. Tamaru, X. Wang, M. Okubo and A. Yamada, Electrochem. Commun., 2013, 33, 23–26 CrossRef CAS.
- J. Zhao, J. Xu, D. H. Lee, N. Dimov, Y. S. Meng and S. Okada, J. Power Sources, 2014, 264, 235–239 CrossRef CAS.
- P. Vassilaras, A. J. Toumar and G. Ceder, Electrochem. Commun., 2014, 38, 79–81 CrossRef CAS.
- A. Darwiche, M. Toiron, M. T. Sougrati, B. Fraisse, L. Stievano and L. Monconduit, J. Power Sources, 2015, 280, 588–592 CrossRef CAS.
- J. Yan, X. Liu and B. Li, Electrochem. Commun., 2015, 56, 46–50 CrossRef CAS.
- L. Baggetto, M. Marszewski, J. Gorka, M. Jaroniec and G. M. Veith, J. Power Sources, 2013, 243, 699–705 CrossRef CAS.
- S. Renault, V. A. Mihali, K. Edstrom and D. Brandell, Electrochem. Commun., 2014, 45, 52–55 CrossRef CAS.
- Y. Zheng, P. Zhang, S. Q. Wu, Y. H. Wen, Z. Z. Zhu and Y. Yang, J. Electrochem. Soc., 2013, 160, A927–A932 CrossRef CAS.
- R. R. Zhao, Y. L. Cao, X. P. Ai and H. X. Yang, J. Electroanal. Chem., 2013, 688, 93–97 CrossRef CAS.
- E. Hosono, T. Saito, J. Hoshino, M. Okubo, Y. Saito, D. Nishio-Hamane, T. Kudo and H. Zhou, J. Power Sources, 2012, 217, 43–46 CrossRef CAS.
- S. Yu, P. Zhang, S. Q. Wu, A. Y. Li, Z. Z. Zhu and Y. Yang, J. Solid State Electrochem., 2014, 18, 2071–2075 CrossRef CAS.
- J. Zhao, L. Zhao, K. Chihara, S. Okada, J.-i. Yamaki, S. Matsumoto, S. Kuze and K. Nakane, J. Power Sources, 2013, 244, 752–757 CrossRef CAS.
- A. Ponrouch and M. R. Palacin, Electrochem. Commun., 2015, 54, 51–54 CrossRef CAS.
- X. Lin, P. Li, L. Shao, X. Zheng, M. Shui, N. Long, D. Wang and J. Shu, Electrochim. Acta, 2015, 169, 382–394 CrossRef CAS.
- R. Zhao, L. Zhu, Y. Cao, X. Ai and H. X. Yang, Electrochem. Commun., 2012, 21, 36–38 CrossRef CAS.
- M. Valvo, F. Lindgren, U. Lafont, F. Bjorefors and K. Edstrom, J. Power Sources, 2014, 245, 967–978 CrossRef CAS.
- A. Langrock, Y. Xu, Y. Liu, S. Ehrman, A. Manivannan and C. Wang, J. Power Sources, 2013, 223, 62–67 CrossRef CAS.
- P. Rozier, M. Sathiya, A.-R. Paulraj, D. Foix, T. Desaunay, P.-L. Taberna, P. Simon and J.-M. Tarascon, Electrochem. Commun., 2015, 53, 29–32 CrossRef CAS.
- J. Qian, Y. Xiong, Y. Cao, X. Ai and H. Yang, Nano Lett., 2014, 14, 1865–1869 CrossRef CAS PubMed.
- F. Zhao, N. Han, W. Huang, J. Li, H. Ye, F. Chen and Y. Li, J. Mater. Chem. A, 2015, 3, 21754–21759 CAS.
- J. Fullenwarth, A. Darwiche, A. Soares, B. Donnadieu and L. Monconduit, J. Mater. Chem. A, 2014, 2, 2050–2059 CAS.
- S. Zhang, Y. Li and N. Pan, J. Power Sources, 2012, 206, 476–482 CrossRef CAS.
- H. Yu, J. Wu, L. Fan, Y. Lin, K. Xu, Z. Tang, C. Cheng, S. Tang, J. Lin, M. Huang and Z. Lan, J. Power Sources, 2012, 198, 402–407 CrossRef CAS.
- M. Huang, F. Li, X. Zhao, D. Luo, X. You, Y. Zhang and G. Li, Electrochim. Acta, 2015, 152, 172–177 CrossRef CAS.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- D. Yan, Z. Guo, G. Zhu, Z. Yu, H. Xu and A. Yu, J. Power Sources, 2012, 199, 409–412 CrossRef CAS.
- G. Xiong, K. P. S. S. Hembram, R. G. Reifenberger and T. S. Fisher, J. Power Sources, 2013, 227, 254–259 CrossRef CAS.
- Y. Yuan, X. Xia, J. Wu, X. Huang, Y. Pei, J. Yang and S. Guo, Electrochem. Commun., 2011, 13, 1123–1126 CrossRef CAS.
- R. Tummala, R. K. Guduru and P. S. Mohanty, J. Power Sources, 2012, 209, 44–51 CrossRef CAS.
- S. Park and S. Kim, Electrochim. Acta, 2013, 89, 516–522 CrossRef CAS.
- Y. Wang, J. Chen, J. Cao, Y. Liu, Y. Zhou, J.-H. Ouyang and D. Jia, J. Power Sources, 2014, 271, 269–277 CrossRef CAS.
- K. Xie, X. Qin, X. Wang, Y. Wang, H. Tao, Q. Wu, L. Yang and Z. Hu, Adv. Mater., 2012, 24, 347–352 CrossRef CAS PubMed.
- M. Yang, B. Cheng, H. Song and X. Chen, Electrochim. Acta, 2010, 55, 7021–7027 CrossRef CAS.
- A. Bello, F. Barzegar, D. Momodu, J. Dangbegnon, F. Taghizadeh, M. Fabiane and N. Manyala, J. Power Sources, 2015, 273, 305–311 CrossRef CAS.
- L. Wang, X. Li, T. Guo, X. Yan and B.-K. Tay, Int. J. Hydrogen Energy, 2014, 39, 7876–7884 CrossRef CAS.
- C. Zheng, X. Zhou, H. Cao, G. Wang and Z. Liu, J. Power Sources, 2014, 258, 290–296 CrossRef CAS.
- W. Yang, Z. Gao, J. Wang, B. Wang, Q. Liu, Z. Li, T. Mann, P. Yang, M. Zhang and L. Liu, Electrochim. Acta, 2012, 69, 112–119 CrossRef CAS.
- B. Zhao, P. Liu, Y. Jiang, D. Pan, H. Tao, J. Song, T. Fang and W. Xu, J. Power Sources, 2012, 198, 423–427 CrossRef CAS.
- T. Xue, X. Wang and J.-M. Lee, J. Power Sources, 2012, 201, 382–386 CrossRef CAS.
- X. Zhang, P. Yu, H. Zhang, D. Zhang, X. Sun and Y. Ma, Electrochim. Acta, 2013, 89, 523–529 CrossRef CAS.
- Y. Yan, Q. Cheng, G. Wang and C. Li, J. Power Sources, 2011, 196, 7835–7840 CrossRef CAS.
- P. Sen and A. De, Electrochim. Acta, 2010, 55, 4677–4684 CrossRef CAS.
- R. Amade, E. Jover, B. Caglar, T. Mutlu and E. Bertran, J. Power Sources, 2011, 196, 5779–5783 CrossRef CAS.
- Y. Zhao, M. Liu, X. Deng, L. Miao, P.-K. Tripathi, X. Ma, D. Zhu, Z. Xu, Z. Hao and L. Gan, Electrochim. Acta, 2015, 153, 448–455 CrossRef CAS.
- D. Bhattacharjya, M.-S. Kim, T.-S. Bae and J.-S. Yu, J. Power Sources, 2013, 244, 799–805 CrossRef CAS.
- A. Izadi-Najafabadi, S. Yasuda, K. Kobashi, T. Yamada, D. N. Futaba, H. Hatori, M. Yumura, S. Iijima and K. Hata, Adv. Mater., 2010, 22, E235–E241 CrossRef CAS PubMed.
- K. Xie, X. Qin, X. Wang, Y. Wang, H. Tao, Q. Wu, L. Yang and Z. Hu, Adv. Mater., 2012, 24, 347–352 CrossRef CAS PubMed.
- Y. Zhang, H. Feng, X. B. Wu, L. Z. Wang, A. Q. Zhang, T. C. Xia, H. C. Dong, X. F. Li and L. S. Zhang, Int. J. Hydrogen Energy, 2009, 34, 4889 CrossRef CAS.
- A. Burke, J. Power Sources, 2000, 91, 37–50 CrossRef CAS.
- J. R. Miller and P. Simon, Science, 2008, 321, 651–652 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- Z. Chen, Y. Qin, D. Weng, Q. Xiao, Y. Peng, X. Wang, H. Li, F. Wei and Y. Lu, Adv. Funct. Mater., 2009, 19, 3420–3426 CrossRef CAS.
- A.-M. Alexander and J. S. J. Hargreaves, Chem. Soc. Rev., 2010, 39, 4388–4401 RSC.
- S. T. Oyama, J. Catal., 2003, 216, 343–352 CrossRef CAS.
- J. Y. Xiang, X. L. Wang, J. Zhong, D. Zhang and J. P. Tu, J. Power Sources, 2011, 196, 379–385 CrossRef CAS.
- Y. Lu, J. P. Tu, J. Y. Xiang, X. L. Wang, J. Zhang, Y. J. Mai and S. X. Mao, J. Phys. Chem. C, 2011, 115, 23760–23767 CAS.
- S. Boyanov, K. Annou, C. Villevieille, M. Pelosi, D. Zitoun and L. Monconduit, Ionics, 2007, 14, 183–190 CrossRef.
- S. Carenco, D. Portehault, C. Boissiere, N. Mezailles and C. Sanchez, Chem. Rev., 2013, 113, 7981 CrossRef CAS PubMed.
- K. Zhou, W. Zhou, L. Yang, J. Lu, S. Cheng, W. Mai, Z. Tang, L. Li and S. Chen, Adv. Funct. Mater., 2015, 25, 7530–7538 CrossRef.
- Y. Lu, J.-k. Liu, X.-Y. Liu, S. Huang, T.-Q. Wang, X.-l. Wang, C.-D. Gu, J.-P. Tu and S. X. Mao, Crystengcomm, 2013, 15, 7071–7079 RSC.
- S. Duan and R. Wang, NPG Asia Mater., 2014, 6, e122 CrossRef CAS.
- W. Du, R. Kang, P. Geng, X. Xiong, D. Li, Q. Tian and H. Pang, Mater. Chem. Phys., 2015, 165, 207–214 CrossRef CAS.
- F. Bella, A. Sacco, D. Pugliese, M. Laurenti and S. Bianco, J. Power Sources, 2014, 264, 333–343 CrossRef CAS.
- F. Bella, G. Griffini, M. Gerosa, S. Turri and R. Bongiovanni, J. Power Sources, 2015, 283, 195–203 CrossRef CAS.
- H.-W. Chen, Y.-D. Chiang, C.-W. Kung, N. Sakai, M. Ikegami, Y. Yamauchi, K. C. W. Wu, T. Miyasaka and K.-C. Ho, J. Power Sources, 2014, 245, 411–417 CrossRef CAS.
- Y. Xue, J. Liu, H. Chen, R. Wang, D. Li, J. Qu and L. Dai, Angew. Chem., Int. Ed., 2012, 51, 12124–12127 CrossRef CAS PubMed.
- B. Hu, Q. Tang, B. He, L. Lin and H. Chen, J. Power Sources, 2014, 267, 445–451 CrossRef CAS.
- J. Yu, Q. Li and Z. Shu, Electrochim. Acta, 2011, 56, 6293–6298 CrossRef CAS.
- D. Kim, P. Roy, K. Lee and P. Schmuki, Electrochem. Commun., 2010, 12, 574–578 CrossRef CAS.
- K. Fan, T. Peng, J. Chen, X. Zhang and R. Li, J. Power Sources, 2013, 222, 38–44 CrossRef CAS.
- H. Xu, X. Tao, D. Wang, Y. Zheng and J. Chen, Electrochim. Acta, 2010, 55, 2280–2285 CrossRef CAS.
- J. E. Benedetti, A. D. Goncalves, A. L. B. Formiga, M.-A. De Paoli, X. Li, J. R. Durrant and A. F. Nogueira, J. Power Sources, 2010, 195, 1246–1255 CrossRef CAS.
- F. Bella, E.-D. Ozzello, A. Sacco, S. Bianco and R. Bongiovanni, Int. J. Hydrogen Energy, 2014, 39, 3036–3045 CrossRef CAS.
- H. Chang, C.-M. Hsu, P.-K. Kao, Y.-J. Yang, C.-C. Hsu, I. C. Cheng and J.-Z. Chen, J. Power Sources, 2014, 251, 215–221 CrossRef CAS.
- J. Zhang, X. Li, W. Guo, T. Hreid, J. Hou, H. Su and Z. Yuan, Electrochim. Acta, 2011, 56, 3147–3152 CrossRef CAS.
- Y. Liu, X. Sun, Q. Tai, H. Hu, B. Chen, N. Huang, B. Sebo and X.-z. Zhao, J. Power Sources, 2011, 196, 475–481 CrossRef CAS.
- C. Bu, Q. Tai, Y. Liu, S. Guo and X. Zhao, J. Power Sources, 2013, 221, 78–83 CrossRef CAS.
- P. Joshi, L. Zhang, Q. Chen, D. Galipeau, H. Fong and Q. Qiao, ACS Appl. Mater. Interfaces, 2010, 2, 3572–3577 CAS.
- H. Cai, Q. Tang, B. He and P. Li, J. Power Sources, 2014, 258, 117–121 CrossRef CAS.
- L.-H. Chang, C.-K. Hsieh, M.-C. Hsiao, J.-C. Chiang, P.-I. Liu, K.-K. Ho, C.-C. M. Ma, M.-Y. Yen, M.-C. Tsai and C.-H. Tsai, J. Power Sources, 2013, 222, 518–525 CrossRef CAS.
- B. He, Q. Tang, J. Luo, Q. Li, X. Chen and H. Cai, J. Power Sources, 2014, 256, 170–177 CrossRef CAS.
- B. He, X. Meng, Q. Tang, P. Li, S. Yuan and P. Yang, J. Power Sources, 2014, 260, 180–185 CrossRef CAS.
- M. S. Wu and J. F. Wu, Chem. Commun., 2013, 49, 10971–10973 RSC.
- M. Wu, J. Bai, Y. Wang, A. Wang, X. Lin, L. Wang, Y. Shen, Z. Wang, A. Hagfeldt and T. Ma, J. Mater. Chem., 2012, 22, 11121–11127 RSC.
- M.-S. Wu, C.-J. Chung and Z.-Z. Ceng, RSC Adv., 2015, 5, 4561–4567 RSC.
- P. Wang, X. Li, Z. Xu, Z. Wu, S. Zhang, W. Xu, H. Zhong, H. Chen, E. Li, J. Luo, Q. Yu and S. Lin, Nano Energy, 2015, 13, 509–517 CrossRef CAS.
- C. C. Chen, M. Aykol, C. C. Chang, A. F. J. Levi and S. B. Cronin, Nano Lett., 2011, 11, 1863–1867 CrossRef CAS PubMed.
- L. Zhang, L. Fan, Z. Li, E. Shi, X. M. Li, H. B. Li, C. Y. Ji, Y. Jia, J. Q. Wei, K. L. Wang, H. W. Zhu, D. H. Wu and A. Y. Cao, Nano Res., 2011, 4, 891–900 CrossRef CAS.
- X. M. Li, H. W. Zhu, K. L. Wang, A. Y. Cao, J. Q. Wei, C. Y. Li, Y. Jia, Z. Li, X. Li and D. H. Wu, Adv. Mater., 2010, 22, 2743–2748 CrossRef CAS PubMed.
- X. Miao, S. Tongay, M. K. Petterson, K. Berke, A. G. Rinzler, B. R. Appleton and A. F. Hebard, Nano Lett., 2012, 12, 2745–2750 CrossRef CAS PubMed.
- E. Z. Shi, H. B. Li, L. Yang, L. H. Zhang, Z. Li, P. X. Li, Y. Y. Shang, S. T. Wu, X. M. Li, J. Q. Wei, K. L. Wang, H. W. Zhu, D. H. Wu, Y. Fang and A. Y. Cao, Nano Lett., 2013, 13, 1776–1781 CAS.
- W. S. Ko, T.-T. D. Tran, I. Bhattacharya, K. W. Ng, H. Sun and C. Chang-Hasnain, Nano Lett., 2015, 15, 4961–4967 CrossRef CAS PubMed.
- M. Feifel, T. Rachow, J. Benick, J. Ohlmann, S. Janz, M. Hermle, F. Dimroth and D. Lackner, IEEE Journal of Photovoltaics, 2016, 6, 384–390 CrossRef.
- Z. Wang, E. S. Brown and S. Maldonado, Chin. Chem. Lett., 2015, 26, 469–473 CrossRef CAS.
- L. Britnell, R. V. Gorbachev, R. Jalil, B. D. Belle, F. Schedin, A. Mishchenko, T. Georgiou, M. I. Katsnelson, L. Eaves, S. V. Morozov, N. M. R. Peres, J. Leist, A. K. Geim, K. S. Novoselov and L. A. Ponomarenko, Science, 2012, 335, 947–950 CrossRef CAS PubMed.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- O. Vazquez-Mena, J. P. Bosco, O. Ergen, H. I. Rasool, A. Fathalizadeh, M. Tosun, M. Crommie, A. Javey, H. A. Atwater and A. Zettl, Nano Lett., 2014, 14, 4280–4285 CrossRef CAS PubMed.
- Z. Huang, Z. Chen, Z. Chen, C. Lv, M. G. Humphrey and C. Zhang, Nano Energy, 2014, 9, 373–382 CrossRef CAS.
- L. Li, X. Li, L. Ai and J. Jiang, RSC Adv., 2015, 5, 90265–90271 RSC.
- E. J. Popczun, C. G. Read, C. W. Roske, N. S. Lewis and R. E. Schaak, Angew. Chem., Int. Ed. Engl., 2014, 53, 5427–5430 CrossRef CAS PubMed.
- H. Yang, Y. Zhang, F. Hu and Q. Wang, Nano Lett., 2015, 15, 7616–7620 CrossRef CAS PubMed.
- J.-S. Moon, J.-H. Jang, E.-G. Kim, Y.-H. Chung, S. J. Yoo and Y.-K. Lee, J. Catal., 2015, 326, 92–99 CrossRef CAS.
- E. J. Popczun, J. R. McKone, C. G. Read, A. J. Biacchi, A. M. Wiltrout, N. S. Lewis and R. E. Schaak, J. Am. Chem. Soc., 2013, 135, 9267–9270 CrossRef CAS PubMed.
- X. Wang, Y. V. Kolen'ko, X.-Q. Bao, K. Kovnir and L. Liu, Angew. Chem., Int. Ed., 2015, 54, 8188–8192 CrossRef CAS PubMed.
- F. J. Callejas, M. J. McEnaney, G. C. Read, J. C. Crompton, J. A. Biacchi, J. E. Popczun, R. T. Gordon, S. N. Lewis and E. R. Schaak, ACS Nano, 2014, 8, 11101–11107 CrossRef PubMed.
- J. M. McEnaney, J. C. Crompton, J. F. Callejas, E. J. Popczun, A. J. Biacchi, N. S. Lewis and R. E. Schaak, Chem. Mater., 2014, 26, 4826–4831 CrossRef CAS.
- P. Xiao, M. A. Sk, L. Thia, X. Ge, R. J. Lim, J.-Y. Wang, K. H. Lim and X. Wang, Energy Environ. Sci., 2014, 7, 2624–2629 CAS.
- J. Tian, Q. Liu, N. Cheng, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed. Engl., 2014, 53, 9577–9581 CrossRef CAS PubMed.
- W. Wu, X. Yue, X.-Y. Wu and C.-Z. Lu, RSC Adv., 2016, 6, 24361–24365 RSC.
- J. Wang, W. Yang and J. Liu, J. Mater. Chem. A, 2016, 4, 4686–4690 CAS.
- Z. Jin, P. Li and D. Xiao, Green Chem., 2016, 18, 1459–1464 RSC.
- Y. Yan, B. Y. Xia, X. Wang, X. Ge, Z. Liu and A. Fisher, Chem.–Eur. J., 2015, 21, 18062–18067 CrossRef CAS PubMed.
- C. Zhu, M. Zheng, Z. Xiong, H. Li and W. Shen, Int. J. Hydrogen Energy, 2014, 39, 10861–10869 CrossRef CAS.
- T. Wu, S. Chen, D. Zhang and J. Hou, J. Mater. Chem. A, 2015, 3, 10360–10367 CAS.
- K. Chen, X. Huang, C. Wan and H. Liu, RSC Adv., 2015, 5, 92893–92898 RSC.
- J. Ma, Y. Tang, G. Yang, Y. Chen, Q. Zhou, T. Lu and J. Zheng, Appl. Surf. Sci., 2011, 257, 6494–6497 CrossRef CAS.
- J. Zhu, G. He and P. K. Shen, J. Power Sources, 2015, 275, 279–283 CrossRef CAS.
- X. Li, H. Wang, H. Yu, Z. Liu, H. Wang and F. Peng, Electrochim. Acta, 2015, 185, 178–183 CrossRef CAS.
- C. Wang, T. Ding, Y. Sun, X. Zhou, Y. Liu and Q. Yang, Nanoscale, 2015, 7, 19241–19249 RSC.
- K. Kubota and S. Komaba, J. Electrochem. Soc., 2015, 162, A2538–A2550 CrossRef CAS.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
| This journal is © The Royal Society of Chemistry 2016 |






