Reaction process for ZnCl2 activation of phenol liquefied wood fibers
Yuxiang Huang†
,
Zhigao Liu† and
Guangjie Zhao*
College of Materials Science and Technology, Beijing Forestry University, Tsinghua East Road 35, Haidian, Beijing, 100083, China. E-mail: zhaows@bjfu.edu.cn; Fax: +86 010 62338358; Tel: +86 010 62338358
First published on 1st August 2016
Abstract
In this study, ZnCl2 activation of phenol liquefied wood fibers at different temperatures was conducted to investigate the reaction mechanisms during the activation process. By combining Fourier transform infrared (FTIR) spectroscopy, thermogravimetric-FTIR-mass spectrometry (TG-FTIR-MS) and elemental analysis, the reaction mechanisms during the ZnCl2 activation were deduced. Below 327 °C, dehydration of the hydroxyl groups and fracturing of molecular chains occurred inside the fiber. At 327–700 °C was the main stage of activation, where molecular structure was rearranged due to intramolecular condensation, crosslinking reactions and scission of molecular chains. A carbon net-like structure was preliminarily formed at 402 °C. Due to the removal of pyrolysis products such as H2, H2O, CO, CO2, benzene and phenol, surface defects were filled in molten ZnCl2. Pores would be left on the fiber surface after activation, cooling and cleaning. Above 700 °C, the aromatization degree and the degree of aromatic ring gradually improved.
1. Introduction
Activated carbon fiber is a kind of functional carbon fiber with abundant engineered porosity, that is produced from carbon fiber or carbonizable fiber via physical activation, chemical activation or a combination of physical and chemical activation. In comparison with commercial activated carbons, it has better pore structure and shape,1 which widely extends its application in fields such as separation/purification of gases and liquids, catalytic processing and supercapacitors.2–4Recently, biomass resources have drawn much attention as precursors to ACFs owing to their abundance, renewability, low price and environmental friendliness5–7 compared with traditional fossil resources such as coal and pitch.8 Wood, as the largest lignocellulosic biomass material, has been successfully used as a precursor to prepare ACFs via steam activation or KOH activation.9–12 Also, the reaction mechanisms during the KOH activation process of liquefied wood-based fibers were investigated in detail. On the basis of this, we successfully fabricated supercapacitors with high electrochemical performance (225 F g−1) based on KOH-activated ACFs from liquefied wood.13 Most recently, ZnCl2 as an activating agent has been favoured by people,14–18 probably because it can make precursors pyrolyze at a lower temperature and with a higher yield.19 Usually, the temperature for steam activation or KOH activation is required to be above 800 °C to obtain abundant microporosity, while ZnCl2 activation at low temperature can achieve this. In our previous work, we just produced liquefied wood-based ACFs with a high specific surface area (1423 m2 g−1) via ZnCl2 activation at 700 °C,20 demonstrating that ZnCl2 is an available activation agent for the preparation of ACFs from liquefied wood. However, nothing was known about the reaction process for ZnCl2 activation of liquefied wood-based fibers. Unlike KOH, ZnCl2 did not react with carbon directly and it was reported to promote a dehydration reaction between the polymers as a catalyst.21 Nevertheless, some papers pointed out that ZnCl2 could be intercalated into the carbon matrix, which led to a severe interaction between the zinc compounds upon pyrolysis. Furthermore, the constitutions and structures of the liquefied wood-based fibers were very complicated due to the multifarious components of wood. Therefore, it was necessary to investigate the reaction process for ZnCl2 activation of liquefied wood fibers.
Herein, in order to accurately master the process for adjusting the pore structure of liquefied wood-based ACFs by ZnCl2 activation, thermogravimetric analysis (TG), Fourier transform infrared spectroscopy (FTIR), TG-FTIR and TG-mass spectrometer (MS) of the samples during the ZnCl2 activation process, as well as their elemental compositions, were examined. On the basis of the spectra information and the relationship between spectra, the possible reaction process during ZnCl2 activation was preliminarily speculated.
2. Experimental
2.1 Materials
Chinese fir (Cunninghamia lanceolate), sourced from Fujian, China, was used as a raw material. Phosphoric acid (H3PO4), phenol (C6H5OH), hydrochloric acid (HCl), hexamethylenetetramine (C6H12N4), formaldehyde (CH2O) and zinc chloride (ZnCl2) were purchased from Beijing Chemical Works. The chemicals were all of reagent grade and used without further purification.2.2 Methods
Precursor fibers were prepared from Chinese fir through a series of processes including liquefaction, melt-spinning, curing and washing.22 2 g of precursor fibers were impregnated in 50 mL of ZnCl2 aqueous solution. The amount of activating agent in such a solution corresponded to a given ZnCl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) precursor fiber ratio (4
precursor fiber ratio (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by weight). After 24 h of impregnation, the fibers were placed into a 103 °C oven for 24 h of drying. Subsequently, ZnCl2-impregnated fibers were placed in a horizontal transparent tube furnace (Y02PB, Thermacraft Inc., USA) and heated from room temperature to a specified temperature (400–900 °C) at a heating rate of 4 °C min−1. The hold time was 1 h. The resultant fibers were washed with 1 mol L−1 HCl, then rinsed several times in distilled water until the pH reached neutral. Finally, the fibers were dried in an oven at 103 °C for 24 h. The as-prepared ACFs were denoted ZT, where T (400, 500, 600, 700, 800, 900) represented the activation temperature.
1 by weight). After 24 h of impregnation, the fibers were placed into a 103 °C oven for 24 h of drying. Subsequently, ZnCl2-impregnated fibers were placed in a horizontal transparent tube furnace (Y02PB, Thermacraft Inc., USA) and heated from room temperature to a specified temperature (400–900 °C) at a heating rate of 4 °C min−1. The hold time was 1 h. The resultant fibers were washed with 1 mol L−1 HCl, then rinsed several times in distilled water until the pH reached neutral. Finally, the fibers were dried in an oven at 103 °C for 24 h. The as-prepared ACFs were denoted ZT, where T (400, 500, 600, 700, 800, 900) represented the activation temperature.
The chemical groups of the precursor fibers and ACFs activated at different temperatures were studied using a Fourier transform infrared spectrometer (Tensor 27, BRUKER, Germany). Pressed potassium bromide pellets containing 5% of the samples were used and the scanning range was 4000–500 cm−1.
The percentage composition of C, H, N elements for all the ACF samples was examined by an element analyzer (AFLASHEA1112, Thermo, USA). The relative percentage content of O was calculated using WO = (1 − WC − WH − WN) × 100%, where WC, WH and WN represented the percentage content of C, H and N, respectively.
The weight loss of the precursor fibers and the ZnCl2-impregnated fibers during the heating process was detected using a TG/DTG/DSC thermal analyzer (NETZSCH STA 449F3, Germany). The temperature range was from 50 to 1000 °C and the heating rate was 20 °C min−1. The thermal analyzer coupled with a quadrupole mass spectrometer and a FTIR spectrometer was used for TG-FTIR-MS measurements to analyze the pyrolysis products during the heating process of ZnCl2-impregnated fibers. The samples were placed in a small crucible and then heated to 1000 °C in a nitrogen atmosphere with a heating rate of 10 °C min−1.
3. Results and discussion
3.1 FTIR spectrum analysis
Fig. 1 shows the 4000–400 cm−1 infrared spectral region of the precursor fibers and ACFs prepared by ZnCl2 activation at different temperatures. The identified functional groups corresponding to the major peaks in the spectra are presented in Table 1. A lot of characteristic peaks appeared on the FTIR spectrum of the precursor fibers, indicating their complicated chemical structure. A broad peak at 3430 cm−1 was attributed to the stretching vibration of a hydroxyl and the peak at 3003 cm−1 was related to the stretching of C–H benzene rings. C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in benzene rings can be identified by three peaks at 1608, 1501 and 1452 cm−1.23 In the region of 900–650 cm−1, the peak at 752 cm−1 was ascribed to disubstituted-1,2 in the aromatic rings,24 while the peaks at 878 and 818 cm−1 corresponded to 1,2,4-substitutions and 1,4-substitutions, respectively. The peaks at 1357, 1219 and 1011 cm−1 were assigned to the stretching vibration of a phenolic hydroxyl group, C–O– and a hydroxymethyl group, respectively. The spectra of the precursor fibers also shows two obvious peaks at 2923 and 2853 cm−1 owing to the stretching vibration of methylene, which may come from –CH2– and CH2OCH2 generated by the condensation and addition reaction between the original fibers and HCHO during the curing process. Another possibility was that the HOH2C+ ions reacted with benzene rings to produce hydroxymethyl groups under acidic conditions. A weak peak at 1735 cm−1 may be attributed to the stretching vibration of C
C in benzene rings can be identified by three peaks at 1608, 1501 and 1452 cm−1.23 In the region of 900–650 cm−1, the peak at 752 cm−1 was ascribed to disubstituted-1,2 in the aromatic rings,24 while the peaks at 878 and 818 cm−1 corresponded to 1,2,4-substitutions and 1,4-substitutions, respectively. The peaks at 1357, 1219 and 1011 cm−1 were assigned to the stretching vibration of a phenolic hydroxyl group, C–O– and a hydroxymethyl group, respectively. The spectra of the precursor fibers also shows two obvious peaks at 2923 and 2853 cm−1 owing to the stretching vibration of methylene, which may come from –CH2– and CH2OCH2 generated by the condensation and addition reaction between the original fibers and HCHO during the curing process. Another possibility was that the HOH2C+ ions reacted with benzene rings to produce hydroxymethyl groups under acidic conditions. A weak peak at 1735 cm−1 may be attributed to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in aryl ketone, ester groups or carbonyl groups.25
O in aryl ketone, ester groups or carbonyl groups.25
| Wavenumber (cm−1) | Peak assignment |
|---|---|
| 3430–3400 | Stretching vibration of OH |
| 3003 | Stretching vibration of aromatic CH |
| 2920 | Asymmetric stretching vibration of CH2 |
| 2852 | Symmetric stretching vibration of CH2 |
| 1735 | Stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
| 1608, 1501, 1452 | Stretching vibration of benzene ring |
| 1470 | Bending vibration of CH2 |
| 1357 | Stretching vibration of phenolic hydroxyl group |
| 1219 | Stretching vibration of C–O |
| 1131 | Stretching vibration of aromatic C–O–C |
| 1011 | Stretching vibration of C–O in methylol |
| 880, 815 | Out-of-plane ring deformation (1,2,4) |
| 870 | Polysubstituted aromatic ring |
| 820 | Out-of-plane ring deformation (1,4) |
| 755 | Out-of-plane ring deformation (1,2) |
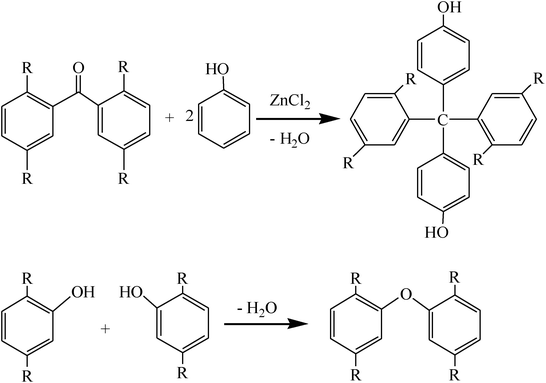
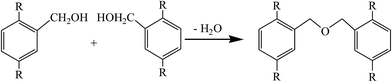
As seen from the FTIR spectrum of Z400, the peak at 3430 cm−1 corresponding to hydroxyl groups became sharper and its position shifted to a high wavenumber when the activation temperature was raised to 400 °C. Meanwhile, the intensity of the peaks at 2920 and 2852 cm−1 corresponding to the stretching vibration of C–H decreased. The peak at 1735 cm−1 corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group disappeared, which can be attributed to ZnCl2, as a typical kind of Lewis acid, catalyzing Friedel–Crafts reactions (reaction (1)) between nucleophilic groups and aromatic hydrocarbon, ketone or aldehyde groups. These reactions of aromatic condensation allowed the evolution of the molecular hydrogen from the hydroaromatic structure of the precursor fiber, leaving the active sites on the adjacent molecules which will undergo the polymerization reactions.26 The disappearance of peaks at 1357, 1219 and 1011 cm−1 and the appearance of a new peak at 1124 cm−1 corresponding to the stretching vibration of aromatic ether bonds indicated that a dehydration condensation reaction (reaction (2)) took place between intramolecular phenolic hydroxyl groups or methylol groups. The variation of peaks in the 900–650 cm−1 region which were characteristic of the out-of-plane bending vibration of C–H in the aromatic rings demonstrated that the cross-linked netlike structure had partly been developed when the precursor fibers were activated at 400 °C. These changes demonstrated that ZnCl2 promoted the catalytic dehydration and condensation reaction between the oxygen containing functional groups in precursor fibers during the heating process from room temperature to 400 °C, which led to the decrease of hydroxyl, methylene, phenolic hydroxyl and methylol groups.
O group disappeared, which can be attributed to ZnCl2, as a typical kind of Lewis acid, catalyzing Friedel–Crafts reactions (reaction (1)) between nucleophilic groups and aromatic hydrocarbon, ketone or aldehyde groups. These reactions of aromatic condensation allowed the evolution of the molecular hydrogen from the hydroaromatic structure of the precursor fiber, leaving the active sites on the adjacent molecules which will undergo the polymerization reactions.26 The disappearance of peaks at 1357, 1219 and 1011 cm−1 and the appearance of a new peak at 1124 cm−1 corresponding to the stretching vibration of aromatic ether bonds indicated that a dehydration condensation reaction (reaction (2)) took place between intramolecular phenolic hydroxyl groups or methylol groups. The variation of peaks in the 900–650 cm−1 region which were characteristic of the out-of-plane bending vibration of C–H in the aromatic rings demonstrated that the cross-linked netlike structure had partly been developed when the precursor fibers were activated at 400 °C. These changes demonstrated that ZnCl2 promoted the catalytic dehydration and condensation reaction between the oxygen containing functional groups in precursor fibers during the heating process from room temperature to 400 °C, which led to the decrease of hydroxyl, methylene, phenolic hydroxyl and methylol groups.
 | (1) |
 | (2) |
When the activation temperature was 400–600 °C, the intensity of the peak at 3430 cm−1 assigned to hydroxyl and the peaks at 950–600 cm−1 attributed to C–H in the aromatic rings27 kept on decreasing, while that of the peaks at 2852 and 2920 cm−1 corresponding to methylene had no obvious changes. In addition, the peak at 1131 cm−1 in the spectrum of Z500 became weak and was shifted to a low wavenumber. In addition, there were few peaks in the region of 1300–1000 cm−1. All of these changes indicated that 400–600 °C was the key temperature range for the activation process, where great changes had occurred in the molecular structure. A large amount of methylol, phenolic hydroxyl and methyl ether bonds connecting benzene rings had been consumed in decomposition, condensation and pyrolysis reactions, which made the non carbon atoms leave the structure in the form of H2O. However, methylene groups remained more due to their better stability.
As the activation temperature further increased (600–800 °C), the intensity of the peak at 3430 cm−1 showed a sharp decrease, illustrating that hydroxyl groups were consumed in dehydration reactions in this temperature section. At the same time, the peaks at 1608 and 1450 cm−1 corresponding to typical vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in benzene rings became weak, indicating large amounts of aromatic rings were changed to multi-benzene fused ring structures with increasing activation temperature. Previous research reported that the multi-benzene fused ring structure was an intermediate state when the structure of carbon materials was changed towards a graphite structure in the process of heat treatment. Therefore, the higher the degree of multi-benzene rings, the higher the degree of polymerization of carbon netlike structures and the higher the degree of graphite-like structures. Nevertheless, there were still some benzene rings in the ZnCl2-activated ACFs, indicating that the activation of precursor fibers below 800 °C was a difficult process for graphitization.
C in benzene rings became weak, indicating large amounts of aromatic rings were changed to multi-benzene fused ring structures with increasing activation temperature. Previous research reported that the multi-benzene fused ring structure was an intermediate state when the structure of carbon materials was changed towards a graphite structure in the process of heat treatment. Therefore, the higher the degree of multi-benzene rings, the higher the degree of polymerization of carbon netlike structures and the higher the degree of graphite-like structures. Nevertheless, there were still some benzene rings in the ZnCl2-activated ACFs, indicating that the activation of precursor fibers below 800 °C was a difficult process for graphitization.
3.2 Element analysis
Table 2 lists the elemental contents of precursor fibers and ZnCl2-activated ACFs at different temperatures. The above FTIR spectra suggested the presence of large amounts of –OH, –CH2OCH2–, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in precursor fibers. Thus, precursor fibers had a high O content (26.15%). The dependence of element (C, H, O) content on activation temperature is presented in Fig. 2. As seen from Fig. 2, carbon content increased rapidly with increasing activation temperature, while hydrogen and oxygen content gradually decreased. The whole process of element variation can be divided into the following four stages.
O in precursor fibers. Thus, precursor fibers had a high O content (26.15%). The dependence of element (C, H, O) content on activation temperature is presented in Fig. 2. As seen from Fig. 2, carbon content increased rapidly with increasing activation temperature, while hydrogen and oxygen content gradually decreased. The whole process of element variation can be divided into the following four stages.
| Samples | Element content (%) | |||
|---|---|---|---|---|
| C | O | H | N | |
| Precursor fibers | 67.85 | 26.15 | 5.29 | 0.71 |
| Z400 | 82.63 | 14.69 | 2.38 | 0.30 |
| Z500 | 83.87 | 13.90 | 1.87 | 0.36 |
| Z600 | 87.35 | 11.04 | 1.29 | 0.32 |
| Z700 | 87.81 | 10.66 | 1.24 | 0.29 |
| Z800 | 88.69 | 10.13 | 0.84 | 0.34 |
| Z900 | 89.91 | 9.14 | 0.67 | 0.28 |
The first stage was from room temperature to 400 °C, where carbon content increased from 67.85% to 82.63%. Meanwhile, oxygen and hydrogen content decreased from 26.15% and 5.29% to 14.69% and 2.38%, respectively. In this stage, the variation of the three elements was the most significant. The main reason was that oxygen containing groups of the ZnCl2-impregnated fibers underwent pyrolysis and dehydration condensation reactions during the heating process below 400 °C, which led to a dramatic decrease in the relative content of oxygen element. Carbon atoms gradually became enriched and began to form a carbon netlike structure.
The second stage was from 400 to 600 °C, which is the main stage for the activation reaction. In this stage, crosslinking condensation reactions continued to take place. Non carbon elements were removed in the form of H2, H2O or other oxygen containing compounds. Accordingly, carbon content increased to 87.35%, while oxygen and hydrogen content decreased to 11.04% and 1.29% respectively.
In the third stage (600–800 °C), carbon content only showed a slight increase. This was because the carbon netlike structure had been initially formed at low temperature. The dehydrogenation and dehydration reactions further occurred in the carbon structure, so hydrogen content kept on decreasing. Meanwhile, oxygen content also decreased in the case of unreacted or newly generated oxygen containing groups, such as carboxyl, which were not stable and decomposed easily at high temperature.
Eventually, the carbon, hydrogen, oxygen content reached 89.91, 0.67 and 9.14%, respectively, after the final stage (800–900 °C).
3.3 TG and DTG analysis
Fig. 3 shows the thermogravimetric (TG) and derivative thermogravimetric (DTG) curves of the precursor fibers and ZnCl2-impreganated fibers. As seen from Fig. 3, there was a big difference in weight loss before and after ZnCl2 impregnation, indicating that ZnCl2, as a dehydrating agent, altered the pyrolysis behavior of carbonaceous materials.28 Combined with Table 3, the pyrolysis process of two samples can be divided into four steps.| Samples | Stages of weight loss | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | |||||||||
| TRa (°C) | WLb (%) | Peak (°C) | TR (°C) | WL (%) | Peak (°C) | TR (°C) | WL (%) | Peak (°C) | TR (°C) | WL (%) | Peak (°C) | |
| a TR: temperature range.b WL: weight loss. | ||||||||||||
| Precursor fibers | 0–150 | 2.7 | — | 150–327 | 7.5 | 284 | 327–800 | 35.8 | 490 | 800–1000 | 0.9 | — |
| ZnCl2-impregnated fibers | 0–150 | 8.5 | 103 | 150–327 | 8 | 284 | 327–700 | 30.1 | 402, 539 | 900–1000 | 7.4 | — |
In the first step, the TG curve of the precursor fibers was changing quite gently and there were no obvious peaks in the DTG curve. For the ZnCl2-impregnated fibers, an obvious decrease can be observed in the TG curve and there was a peak around 103 °C in the DTG curve corresponding to a weight loss of 8.5 wt%. This weight loss can be attributed to the removal of water physically adsorbed by ZnCl2 attached to the fiber surface after impregnation.
In the second step, both the precursor fibers and ZnCl2-impregnated fibers showed less weight loss, corresponding to 7.5 and 8.0 wt%, respectively. There was a peak near 284 °C in both of their DTG curves. For the precursor fibers in this stage, the thermal decomposition was mainly due to the separation of –CH2 in the benzene, breaking of C–O– and release of residual free phenol. The weight loss of ZnCl2-impregnated fibers may have come from the removal of many non carbon elements in the form of byproducts such as H2O.
In the third step, 327–800 °C and 327–700 °C were the main ranges of weight loss for precursor fibers and ZnCl2-impregnated fibers, respectively, according to the TG curves and the variation of peak shape in the DTG curves. There was a peak around 490 °C in the DTG curve of the precursor fibers with a weight loss of 35.8 wt%. However, the TG-DTG curve of ZnCl2-impreganted fibers was quite different to that of the precursor fibers, where two peaks appeared at 402 and 539 °C. This indicated that the addition of ZnCl2 changes the pyrolysis process in precursor fibers. In addition, it can be speculated that ZnCl2 activation started in this temperature range. ZnCl2 catalyzed aromatic condensation reactions, releasing oxygen and hydrogen atoms in the form of water instead of oxygenated organic compounds to promote the formation of aromatic compounds.29 This was the reason why ZnCl2-impregnated fibers had less mass loss (30.1%) than precursor fibers (35.8%).
In the final step, there was little weight loss for precursor fibers, demonstrating that a more stable carbon netlike structure had been formed in the fiber, while some weight loss can still be observed for ZnCl2-impregnated fibers. This may be because dehydration and dehydrogenation reactions continued to take place in the aromatic ring structure to form netlike structures of hexatomic rings. The decomposition of oxygen containing groups at high temperatures can also lead to weight loss. However, the main reason for the mass loss of ZnCl2-impregnated fibers during this stage was because of gasification of ZnCl2, which had a boiling point around 732 °C.30
3.4 TG-FTIR analysis
Fig. 4 shows the three dimensional FTIR spectrum of the gas evolved during the thermal degradation of the ZnCl2-impregnated fiber, where x, y, z coordinates are the wavenumber, absorbance and activation temperature respectively. As seen in the spectrum, there was evolution of H2O at the initial stage of the heating process, which came from the physically adsorbed H2O by ZnCl2. Afterwards, a large amount of gaseous byproducts evolved. With increasing temperature, the peak intensity of evolved gas materials gradually increased. According to the above result of weight loss, the FT-IR spectra of evolved gas at temperatures of 120, 284, 402 and 539 °C were selected for analysis.The FTIR spectrum of the evolved gas for ZnCl2-impregnated fibers activated at 120 °C is shown in Fig. 5a. The peaks at 3964–3480 cm−1 and 1800–1300 cm−1 were the characteristic peaks of H2O. At this temperature there were no characteristic peaks of other substances, illustrating that the pyrolysis product for ZnCl2 activation was only H2O at 120 °C.
Fig. 5b presents the FTIR spectrum of the evolved gas for ZnCl2-impregnated fibers activated at 284 °C. The peaks at 3964–3500 cm−1 were still the characteristic peaks of H2O. The peaks that appeared at 2400–2250 cm−1 and 750–650 cm−1 were attributed to CO2. A peak near 2174 cm−1 corresponded to CO. Two peaks at 3016 and 1508 cm−1 were related to CH4 and H2O, respectively.31 Strong peaks were observed at 1850–1600 cm−1, which were ascribed to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. According to Fig. 7c, it can be speculated that the pyrolysis products at this temperature may contain HCHO. The possible reaction is shown in reaction (3). Meanwhile, weak bands also appeared at 1300–1000 cm−1, which were the characteristic peaks of C–O, indicating that ether or alcohol was generated. Combined with the MS spectrum (m/z = 32), this alcohol was identified as CH3OH. Therefore, the main pyrolysis products were CO, CH4, CO2, H2O, HCHO and CH3OH at 284 °C. Fig. 5c shows the FTIR spectrum at 402 °C. Compared with Fig. 5b, the peak near 2174 cm−1 corresponding to CO has disappeared in Fig. 5c. So the main pyrolysis products were H2O, CO2, CH4 and CH3OH at 402 °C.
O. According to Fig. 7c, it can be speculated that the pyrolysis products at this temperature may contain HCHO. The possible reaction is shown in reaction (3). Meanwhile, weak bands also appeared at 1300–1000 cm−1, which were the characteristic peaks of C–O, indicating that ether or alcohol was generated. Combined with the MS spectrum (m/z = 32), this alcohol was identified as CH3OH. Therefore, the main pyrolysis products were CO, CH4, CO2, H2O, HCHO and CH3OH at 284 °C. Fig. 5c shows the FTIR spectrum at 402 °C. Compared with Fig. 5b, the peak near 2174 cm−1 corresponding to CO has disappeared in Fig. 5c. So the main pyrolysis products were H2O, CO2, CH4 and CH3OH at 402 °C.
 | (3) |
Fig. 5d is the FTIR spectrum of the evolved gas at 539 °C. The characteristic peak of CH4 (3016 cm−1) disappeared and it no longer appeared on increasing the activation temperature, suggesting that CH4 was mainly generated under 500 °C. The peak related to CO reappeared at 2174 cm−1. According to the DTG curve, the thermal reaction rate at 402 °C was different from that at 284 °C, demonstrating that different crosslinking condensation reactions took place in the two stages. Thus, the main evolved gases were CO, CO2, HCHO and H2O.
Fig. 6 shows the FTIR spectra of the evolved gas from 628 to 1000 °C. The main pyrolysis products were CO, CO2 and H2O when the temperature was 600–680 °C. When the temperature continued to rise, the amount of the evolved CO became less after 900 °C.
3.5 TG-MS analysis
In Fig. 7, the evolved gas MS signal plot measured by the TG-MS technique is shown in arbitrary units (a.u.) during the thermal degradation of ZnCl2-impregnated fibers. The main gases were H2 (m/z = 2), H2O (m/z = 18), CO (m/z = 28), HCHO (m/z = 32), CO2 (m/z = 44), benzene (m/z = 78), toluene (m/z = 92), phenol (m/z = 94), cresol (m/z = 108) and dimethyl phenol (m/z = 122).As seen in Fig. 7a, the strongest peak was around 120 °C in the H2O MS curve, which was ascribed to physical desorption of water. Another peak appeared near 400 °C. Meanwhile, there was an adjacent peak at 280 °C, which was consistent with the DTG curve in TG analysis. This indicated that different dehydration condensation, crosslinking reactions took place between 150–327 °C and 327–600 °C, which decreased oxygen containing groups such as hydroxyl and phenolic hydroxyl. When the temperature was above 600 °C, the amount of released H2O increased, which was consistent with a weak intensity of the hydroxyl peak at 3400 cm−1. This demonstrated that dehydration reactions still occurred at high temperatures.
Fig. 7b shows MS responses of H2, CO and CO2 in TG-MS analysis of ZnCl2-impregnated fibers. H2 was mainly generated at 350–1000 °C, with two peaks at 500 and 700 °C, indicating that different dehydrogenation reactions occurred in the two stages. Compared with H2, there was evolution of CO and CO2 during the whole activation process. However, their amounts were very low. This result implied that the byproducts during ZnCl2 activation were dominated by H2 and H2O.
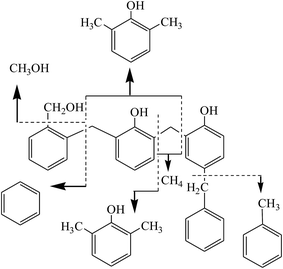
The MS response of evolved CH3OH is presented in Fig. 7c. It was mainly generated below 527 °C, with two maxima at 200 and 400 °C. The release amount of CH3OH was very little when the temperature was above 539 °C, which may come from the abscission of hydroxymethyl groups. Fig. 7d shows the MS response of benzene, toluene, phenol, cresol and dimethyl phenol. The curves of these substances were flat and there were no obvious sharp peaks. They were mainly generated from 300 to 700 °C due to the scission of molecular chains at different locations. The possible reaction is shown in reaction (4). According to these results, it can be speculated that, in addition to macromolecular substances such as benzene, toluene, phenol, cresol and dimethyl phenol, the main pyrolysis byproducts were H2O, H2, CO2, CH3OH and CO.
 | (4) |
3.6 Reaction process during ZnCl2 activation
The ZnCl2 activation process of liquefied wood-based fiber below 900 °C can be basically divided into three stages as follows, combining TG, TG-MS-FTIR, element analysis and FTIR analysis.The first stage was below 327 °C, where the total weight loss was small except for the loss caused by removal of absorbed water around 100 °C. The main reactions were the condensation reaction of intramolecular oxygen containing groups and the fracture of molecular bonds, leading to a decrease of methylol and phenolic hydroxyl groups. In this stage, the pyrolysis byproducts were mainly small molecules such as CH4, HCHO, CH3OH, H2O, CO and CO2.
The second stage was 327–700 °C, which was the main weight loss range during the whole ZnCl2 activation process. In the low temperature range of 327–461 °C, dehydration reactions took place between the hydroxymethyl in the benzene ring and phenolic hydroxyl groups to generate aryl ether bonds and dimethyl ether bonds. Decomposition reactions of molecular chains generated substances such as benzene and phenol. The Friedel–Crafts reaction of arenes promoted the fusing of aromatic rings to rearrange the molecular structure, thereby initially forming a carbon netlike structure at 402 °C. The newly generated ether bonds broke again with increasing temperature. The pyrolysis products in this stage contained H2, H2O, CO and CO2 in addition to tiny amounts of CH4 and CH3OH. The role of ZnCl2 during the activation process was mainly in catalytic dehydration. It started a hot-melting phase transition at 237–411 °C and the swelling effects of the ZnCl2 occurred by lateral bond breaking in the molecules leading to increased inter- and intra-voids. The interspaces between carbon layers created by ZnCl2 would develop the microporosity after ZnCl2 was washed away by HCl and distilled water.26 Meanwhile, micropores were generated by removing the low-molecular-weight volatile compounds from the matrix structure and the gradual vaporization of ZnCl2 when the temperature was increased.
In the final stage (700–900 °C), the weight loss became less. The aromatization degree and the degree of fusing among rings gradually increased with increasing the temperature. The carbon netlike structure changed again. Moreover, at high temperature, newly generated oxygen containing groups such as carboxyl were unstable to decomposition. The main pyrolysis products in this stage included H2, H2O, CO and CO2.
4. Conclusions
In this study, the reaction process for ZnCl2 activation of phenol liquefied wood fibers was studied in detail based on FTIR, TG-FTIR-MS, and element analysis. Carbonization and ZnCl2 activation of liquefied wood-based fibers were going on at the same time during the one-step activation process. When the temperature was below 327 °C, dehydration of hydroxyl groups and fracture of molecular chains occurred inside the fiber. Pyrolysis products such as CH4, HCHO, CH3OH and H2O were found. When the temperature was in the range of 327–700 °C, molecular structure was rearranged due to intramolecular condensation, crosslinking reactions and scission of molecular chains. A carbon netlike structure was preliminarily formed at the beginning of this stage. More products were found, such as H2, H2O, CO, CO2, benzene, phenol, etc. and the level of aromatization and the degree of aromatic ring kept on increasing when the temperature was above 700 °C.Acknowledgements
The authors gratefully acknowledge the financial support from the China National Science & Technology Pillar Program through the project “Key technology and application demonstration for the production of wood-based functional adsorption materials” (2015BAD14B06); and from the China Scholarship Council.References
- A. G. Pandolfo and A. F. Hollenkamp, Carbon properties and their role in supercapacitors, J. Power Sources, 2006, 157, 11–27 CrossRef CAS
.
- X. Du, W. Zhao, Y. Wang, C. Wang, M. Chen, T. Qi, C. Hua and M. Ma, Preparation of activated carbon hollow fibers from ramie at low temperature for electric double-layer capacitor applications, Bioresour. Technol., 2013, 149, 31–37 CrossRef CAS PubMed
.
- S. Aber, A. Khataee and M. Sheydaei, Optimization of activated carbon fiber preparation from kenaf using K2HPO4 as chemical activator for adsorption of phenolic compounds, Bioresour. Technol., 2009, 100, 6586–6591 CrossRef CAS PubMed
.
- B. Xu, F. Wu, R. Chen, G. Cao, S. Chen and Y. Yang, Mesoporous activated carbon fiber as electrode material for high-performance electrochemical double layer capacitors with ionic liquid electrolyte, J. Power Sources, 2010, 195, 2118–2124 CrossRef CAS
.
- K. Song, Q. Wu, Z. Zhang, S. Ren, T. Lei, I. I. Negulescu and Q. Zhang, Porous carbon nanofibers from electrospun biomass tar/polyacrylonitrile/silver hybrids as antimicrobial materials, ACS Appl. Mater. Interfaces, 2015, 7, 15108–15116 CAS
.
- J. Zheng, Q. Zhao and Z. Ye, Preparation and characterization of activated carbon fiber (ACF) from cotton woven waste, Appl. Surf. Sci., 2014, 299, 86–91 CrossRef CAS
.
- M. C. Ncibi, R. Ranguin, M. J. Pintor, V. Jeanne-Rose, M. Sillanp and S. Gaspard, Preparation and characterization of chemically activated carbons derived from Mediterranean Posidonia oceanica (L.) fibres, J. Anal. Appl. Pyrolysis, 2014, 109, 205–214 CrossRef CAS
.
- X. He, R. Li, J. Qiu, K. Xie, P. Ling, M. Yu, X. Zhang and M. Zheng, Synthesis of mesoporous carbons for supercapacitors from coal tar pitch by coupling microwave-assisted KOH activation with a MgO template, Carbon, 2012, 50, 4911–4921 CrossRef CAS
.
- Z. Jin, X. Yan, Y. Yu and G. Zhao, Sustainable activated carbon fibers from liquefied wood with controllable porosity for high-performance supercapacitors, J. Mater. Chem. A, 2014, 2, 11706–11715 CAS
.
- Y. Huang, E. Ma and G. Zhao, Optimizing the pore structure of bio-based ACFs through a simple KOH-steam reactivation, Materials, 2016, 9, 432 CrossRef
.
- Y. Huang and G. Zhao, A novel method for the production of mesoporous activated carbon fibers from liquefied wood, Mater. Lett., 2016, 178, 190–192 CrossRef CAS
.
- Y. Huang, E. Ma and G. Zhao, Preparation of liquefied wood-based activated carbon fibers by different activation methods for methylene blue adsorption, RSC Adv., 2015, 5, 70287–70296 RSC
.
- Y. Huang, L. Peng, Y. Liu, G. Zhao, J. Chen and G. Yu, Biobased nano porous active carbon fibers for high-performance supercapacitors, ACS Appl. Mater. Interfaces, 2016, 8, 15205–15215 CAS
.
- Y. Liu, Y. Wang, G. Zhang, W. Liu and D. Wang, Preparation of activated carbon from willow leaves and evaluation in electric double-layer capacitors, Mater. Lett., 2016, 176, 60–63 CrossRef CAS
.
- Y. Li, Y. Li, L. Li, X. Shi and Z. Wang, Preparation and analysis of activated carbon from sewage sludge and corn stalk, Adv. Powder Technol., 2016, 27, 684–691 CrossRef
.
- H. L. Say and F. Güzel, High surface area mesoporous activated carbon from tomato processing solid waste by zinc chloride activation: process optimization, characterization and dyes adsorption, J. Cleaner Prod., 2016, 113, 995–1004 CrossRef
.
- A. Kumar and H. Mohan Jena, High surface area microporous activated carbons prepared from fox nut (Euryale ferox) shell by zinc chloride activation, Appl. Surf. Sci., 2015, 356, 753–761 CrossRef CAS
.
- J. Wei, H. Ding, Y. Wang and H. Xiong, Hierarchical porous carbon materials with high capacitance derived from Schiff-base networks, ACS Appl. Mater. Interfaces, 2015, 7, 5811–5819 CAS
.
- X. Du, W. Zhao, S. Ma, M. Ma, T. Qi, Y. Wang and C. Hua, Effect of ZnCl2 impregnation concentration on the microstructure and electrical performance of ramie-based activated carbon hollow fiber, Ionics, 2016, 22, 545–553 CrossRef CAS
.
- Z. Liu, Y. Huang and G. Zhao, Preparation and characterization of activated carbon fibers from liquefied wood by ZnCl2 activation, BioResources, 2016, 11, 3178–3190 CAS
.
- Z. Yue, C. L. Mangun and J. Economy, Preparation of fibrous porous materials by chemical activation 1. ZnCl2 activation of polymer-coated fibers, Carbon, 2002, 40, 1181–1191 CrossRef CAS
.
- Y. Huang and G. Zhao, Preparation and characterization of activated carbon fibers from liquefied wood by KOH activation, Holzforschung, 2016, 70, 195–202 CrossRef CAS
.
- Y. Huang, E. Ma and G. Zhao, Thermal and structure analysis on reaction mechanisms during the preparation of activated carbon fibers by KOH activation from liquefied wood-based fibers, Ind. Crops Prod., 2015, 69, 447–455 CrossRef CAS
.
- C. Morterra and M. J. D. Low, I.R. studies of carbon—VII. The pyrolysis of a phenol–formaldehyde resin, Carbon, 1985, 23, 525–530 CrossRef CAS
.
- M. Moniruzzaman and T. Ono, Separation and characterization of cellulose fibers from cypress wood treated with ionic liquid prior to laccase treatment, Bioresour. Technol., 2013, 127, 132–137 CrossRef CAS PubMed
.
- M. A. Yahya, Z. Al-Qodah and C. W. Z. Ngah, Agricultural bio-waste materials as potential sustainable precursors used for activated carbon production: a review, Renewable Sustainable Energy Rev., 2015, 46, 218–235 CrossRef CAS
.
- J. C. Domínguez, M. Oliet, M. V. Alonso, E. Rojo and F. Rodríguez, Structural, thermal and rheological behavior of a bio-based phenolic resin in relation to a commercial resol resin, Ind. Crops Prod., 2013, 42, 308–314 CrossRef
.
- V. Boonamnuayvitaya, S. Sae-ung and W. Tanthapanickakoon, Preparation of activated carbons from coffee residue for the adsorption of formaldehyde, Sep. Purif. Technol., 2005, 42, 159–168 CrossRef CAS
.
- J. S. Macedo, L. Otubo, O. P. Ferreira, I. F. Gimenez, I. O. Mazali and L. S. Barreto, Biomorphic activated porous carbons with complex microstructures from lignocellulosic residues, Microporous Mesoporous Mater., 2008, 107, 276–285 CrossRef CAS
.
- Z. Hu, M. P. Srinivasan and Y. Ni, Novel activation process for preparing highly microporous and mesoporous activated carbons, Carbon, 2001, 39, 877–886 CrossRef CAS
.
- A. M. Mocanu, C. Moldoveanu, L. Odochian, C. M. Paius, N. Apostolescu and R. Neculau, Study on the thermal behavior of casein under nitrogen and air atmosphere by means of the TG-FTIR technique, Thermochim. Acta, 2012, 546, 120–126 CrossRef CAS
.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2016 |







