Photo-electrochemical properties and electronic band structure of kesterite copper chalcogenide Cu2–II–Sn–S4 (II = Fe, Co, Ni) thin films†
Abstract
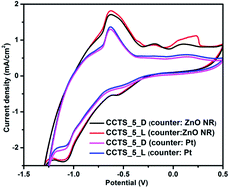
Herein, we report a spin coating route for the deposition of multifunctional quaternary copper chalcogenide Cu2–II–Sn–S4 (II = Fe, Co and Ni) thin films on ITO glass substrates. The surface morphology of the thin films is a uniform porous like structure with an average film thickness of 1.5 μm. The formation of single phase Cu2FeSnS4 (CFTS), Cu2CoSnS4 (CCTS) and Cu2NiSnS4 (CNTS) thin films was confirmed by Raman spectroscopy, which reveals two principal Raman peaks of the A1 phonon mode. Due to the presence of the d6, d7 and d8 electronic configurations within Fe2+, Co2+ and Ni2+ ions, respectively, all the materials show a spin magnetic moment per ion of 3.33 μB, 2.45 μB and 1.31 μB for CFTS, CCTS and CNTS, respectively, as described by the electronic band structure calculation (WIEN2k code). All the thin films have estimated band gap values of 1.87 eV, 1.57 eV and 1.74 eV, respectively, with enhanced photocurrent properties and electrocatalytic activity. Significantly improved charge transfer properties of the thin films were observed from electrochemical impedance spectroscopy analysis under dark and visible light illumination conditions. These thin films are promising for use in photoelectrochemical and solar cell applications.

 Please wait while we load your content...
Please wait while we load your content...