Tunable nanocrystalline CoFe2O4 isotropic powders obtained by co-precipitation and ultrafast ball milling for permanent magnet applications
Abstract
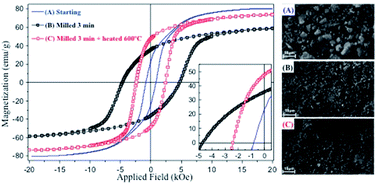
Synthesis of nanocrystalline Co-ferrite powders with tunable magnetic properties is demonstrated by using co-precipitation and a novel ultrafast milling route. Milling times as short as a few minutes are reported for the first time to be sufficient to refine microstructure and to induce microstrain, and act efficiently, providing a 5-fold increase in coercivity. This is achieved with no compositional change during processing, but exclusively through microstructural modification. The efficiency of this process and its feasible scalability pave the way for development of Co-ferrite powders for permanent magnet applications.

 Please wait while we load your content...
Please wait while we load your content...