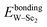The effects of nonmetal dopants on the electronic, optical, and catalytic performances of monolayer WSe2 by a first-principles study†
S. Lua,
C. Li*a,
Y. F. Zhaoa,
H. H. Lia,
Y. Y. Gonga,
L. Y. Niua,
X. J. Liua and
T. Wangb
aInstitute of Coordination Bond Metrology and Engineering, College of Materials Science and Engineering, China Jiliang University, Hangzhou 310018, China. E-mail: canli1983@gmail.com
bCollege of Electrical Engineering, Zhejiang University, Hangzhou 310027, China
First published on 22nd November 2016
Abstract
Doping modifies the electronic, optical, and catalytic behavior of materials through the newly formed chemical bonds and the localized electrons. With the aid of first-principles calculations, the electronic, optical, and catalytic performances of the nonmetal (NM = H, B, C, N, O, F, Si, P, S, Cl, As, Br, Te, or I)-doped monolayer WSe2 were investigated. The results showed that the NM dopants substitute preferentially for Se under a W-rich condition and H, F, Cl, Br, and I atoms are willing to locate at the interstitial site. The electron-clouds around the dopants and nearby W or Se atoms were altered by the newly formed W–NM or Se–NM bonds, with the differences determined by the bonding strength between them. The band gap, optical absorption edge, and intensities were altered or shifted by less than 0.08 eV, 32 nm, and 9.5%, respectively. The H, F, P, Cl, As, Br, and I dopants were conductive to separating the photogenerated e−/h+ pairs, whereas the B, C, Si, and Te dopants became recombination centers for the photogenerated e−/h+ pairs. Compared with pristine monolayer WSe2, NM atoms with odd free electrons reduced the reduction potential by 0.39–0.71 eV and enhanced the oxidation potential by 0.45–0.75 eV. Thus, we can adjust the redox potentials of monolayer WSe2 by introducing different kinds of NM impurities for various catalytic reactions, and the H-, F-, P-, Cl-, As-, Br-, and I-doped specimens have excellent photocatalysis capability.
Introduction
Transition-metal dichalcogenide compounds with a layer structure have attracted considerable attention due to their wide range of electronic, optical, and catalytic performances.1–3 This category of compounds have a sandwiched structure with one transition-metal layer coupled to two chalcogen layers, with the transition-metal atoms bonded to chalcogen atoms by strong covalent interactions and the adjacent sandwiched layers connected to each other by weak van der Waals forces.4–7 Hence, two-dimensional transition-metal dichalcogenide compounds can be easily synthesized with a high specific surface area and with excellent physical and chemical performances. In this regard, monolayer tungsten diselenide (WSe2) has a narrow direct band gap (1.60 eV) and potential applications in electronic devices, optical devices, and as a catalyst.8–11 For instance, high performance field-effect transistors,12 valleytronics,13 photovoltaic devices,14–16 and catalysts11 have been successfully fabricated.To date, the physical and chemical performances of two dimensional WSe2 have been modulated by strain,17 adsorbed molecules,18,19 interface composites,20 vacancies,21 and impurities.22–24 In recent years, several reports have indicated that metal and nonmetal (NM) dopants can effectively alter the electronic,25–27 magnetic,28 optical,29 and catalytic30 performances of monolayer WSe2 by introducing new chemical bonds and changing the binding energies of the nearby bonds. For instance, Hu et al.31 have demonstrated that Re-doped WSe2 can enhance the electronic and optical performances. Kriener et al.24 studied the modification of the electronic structure and the hole-doping effect for the layered WSe2 with a multi-valley band structure, where a Te dopant was found to change the electronic states. Huang et al.32 synthesized a large area S-doped monolayer WSe2 with a tunable band gap, which exhibited promising stable electronic properties. Zou et al.33 reported the excellent hydrogen evolution reaction performance of layered WSe2 by optimizing the substitution of Se with S. Although the physical and chemical performances of several doped layered WSe2 compounds have been studied theoretically and experimentally, the detail on the bond relaxations of the doped specimens is ambivalent. It was reported that chemical bonds not only decide the electronic and optical performances but also affect the redox potential of materials and can be altered by bond relaxation.34 Thus, a detailed study of the chemical bonds in doped WSe2 is necessary to understand the fundamental mechanism of bond relaxation.
In the present work, the structural and thermal performances of monolayer WSe2 after doping with an NM atom (H, B, C, N, O, F, Si, P, S, Cl, As, Br, Te, or I) at the substitutive and interstitial sites were calculated. Also, the electronic and optical performances of all the specimens with a stable configuration were determined by first-principles calculations. Then, their catalytic performances and redox potentials were considered, and the effects of the different NM dopants on the electronic performances and chemical bonds of W–NM or Se–NM were compared.
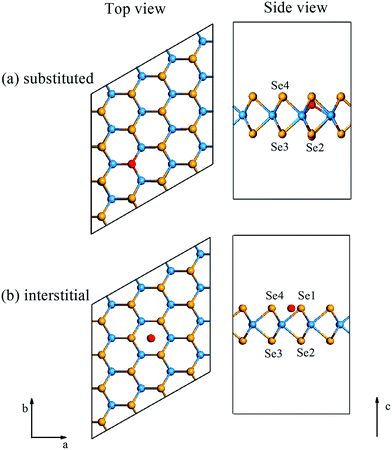
Computational methods
After cutting the bulk WSe2 along its (001) direction, monolayer WSe2 was built. The spurious interactions effect in the nonperiodic directions was considered, whereby a vacuum region of 20 Å was shown to be sufficient. Then, 4 × 4 supercell monolayer W16Se32 after doping with a NM atom at the substitutive and interstitial positions was constructed (see Fig. 1). For the substitutive systems, one Se atom was replaced by one NM atom. Although several doped systems were not neutral due to the different number of valence electrons from the Se atom, the larger distance between the nearest neighbor dopants (about 13.50 Å) was enough to avoid unphysical charge–charge interactions. The density functional theory calculations, including geometry relaxation the and electronic performances [band structure, partial density of states (PDOS), electron density difference, the highest occupied molecular orbital (HOMO), and the lowest unoccupied molecular orbital (LUMO)], were performed utilizing the CASTEP package.35 The generalized gradient approximation (GGA)36 with the Perdew–Wang 91 (PW91) functional and norm-conserving pseudopotentials37 was applied as the exchange–correlation functional. A 720 eV cutoff energy and 7 × 7 × 1 k-point sampling set were used for the convergence. Both the size and atomic positions of the supercells were optimized with a fixed c-axis, and the force and energy tolerances were respectively set as 0.01 eV Å−1 and 1.0 × 10−5 eV per atom, while the maximum displacement was set as 1.0 × 10−3 Å. Moreover, the ab initio molecular dynamics (MD) simulation as implemented in VASP38,39 was carried out for all the substitutive (W64Se124NM4) and interstitial (W64Se128NM4) specimens to explore the stable structure. The simulations were performed in the NVT ensemble with a target temperature of 300 K, where the time step and total time were set as 3 fs and 5 ps, respectively.The substituted Esub and interstitial Eint energies of all the doped systems were defined by the following equation22 to estimate the thermal stability:
 | (1) |
| Ef(WSe2) = EWSe2 − EW − 2ESe | (2) |
Considering the dynamic growth process of WSe2, the atomic chemical potential depends on the growth condition (i.e., when changing from a W-rich condition to a Se-rich condition). Hence, under W-rich conditions, μW was assumed to be the energy per atom in bulk W, and thus μSe could be obtained according to following equation:
| 2μSe + μW = μWSe2 | (3) |
The bonding strengths of the W–NM and W–Se2 bonds of all the doped systems were, respectively, defined as:
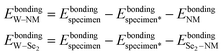 | (4) |
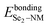 denote the bonding energies of the NM atom and the Se2–NM group.
denote the bonding energies of the NM atom and the Se2–NM group.
Result and discussion
Structural and thermal performances
After geometry relaxation, the lattice parameter (a = 3.298 Å) of WSe2 was consistent with the experimental (3.282 Å (ref. 40) and 3.260 Å (ref. 41)) and other simulated results (3.282 Å (ref. 42)); this ensured the rationality of the calculated parameters. After doping with NM atoms, the crystal configurations of the doped specimens were changed. The lattice parameter a and the bond lengths of the W–NM, Se1–NM, W–Se2, W–Se3, and W–Se4 doped specimens are displayed in Fig. 1 and listed in Table 1. The results show that the bond lengths of the W–NM bonds (dW–NM) of the substituted systems and of the Se–NM bonds (dSe1–NM) of the interstitial systems were changed synchronously, caused by the atomic radius of the NM dopants. Therefore, the nearest neighbor chemical bonds (W–Se2, W–Se3, and W–Se4 bonds) were relaxed by the newly formed W–NM or Se–NM bonds (see Table 1), and the specific values of the W–Se bonds after (ddopedW–Se) and before (dpristineW–Se) doping with the NM atoms are shown in Fig. 2, where the positive and negative values, respectively, denote the bond extension and shrinkage. It was found that all the W–Se bonds had been slightly relaxed and their deformations were less than ±2%. Besides, the deformations of lattice parameters of all the specimens were less than ±1%. Briefly, this shows that the NM dopants affect the crystal configurations of monolayer WSe2 to some extent.| asub | aint | dW–NM | dSe1–NM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pristine | 3.298 | — | — | 2.596 | ||||||
| H | 3.280 | 3.310 | 2.079 | 1.573 | 2.594 | 2.616 | 2.581 | 2.589 | 2.596 | 2.603 |
| B | 3.296 | 3.309 | 2.174 | 2.104 | 2.618 | 2.618 | 2.589 | 2.597 | 2.588 | 2.602 |
| C | 3.292 | 3.331 | 2.071 | 1.870 | 2.643 | 2.601 | 2.599 | 2.623 | 2.609 | 2.606 |
| N | 3.286 | 3.309 | 2.051 | 1.882 | 2.636 | 2.596 | 2.603 | 2.599 | 2.620 | 2.591 |
| O | 3.278 | 3.211 | 2.119 | 1.925 | 2.610 | 2.570 | 2.588 | 2.601 | 2.610 | 2.587 |
| F | 3.287 | 3.211 | 2.313 | 2.482 | 2.596 | 2.581 | 2.575 | 2.588 | 2.594 | 2.578 |
| Si | 3.311 | 3.211 | 2.443 | 2.259 | 2.630 | 2.615 | 2.587 | 2.593 | 2.587 | 2.592 |
| P | 3.296 | 3.211 | 2.475 | 2.183 | 2.601 | 2.578 | 2.604 | 2.591 | 2.603 | 2.583 |
| S | 3.293 | 3.112 | 2.464 | 2.321 | 2.600 | 2.578 | 2.596 | 2.579 | 2.598 | 2.552 |
| Cl | 3.297 | 3.217 | 2.569 | 3.079 | 2.601 | 2.578 | 2.585 | 2.569 | 2.597 | 2.590 |
| As | 3.297 | 3.211 | 2.620 | 2.269 | 2.587 | 2.581 | 2.601 | 2.582 | 2.598 | 2.584 |
| Br | 3.301 | 3.211 | 2.708 | 3.212 | 2.600 | 2.586 | 2.589 | 2.586 | 2.599 | 2.581 |
| Te | 3.265 | 3.211 | 2.783 | 2.442 | 2.585 | 2.581 | 2.592 | 2.575 | 2.590 | 2.589 |
| I | 3.307 | 3.211 | 2.892 | 3.454 | 2.596 | 2.572 | 2.590 | 2.613 | 2.598 | 2.610 |
 | ||
| Fig. 2 Rates of bond length change of the: (a) W–Se1 bond, (c) W–Se2 bond, and (e) W–Se3 bond; rates of lattice constant change of the: (b) W–Se1 bond, (d) W–Se2 bond, and (f) W–Se3 bond. | ||
After the MD simulations, most of the doped specimens were preserved even after running at 5 ps, and the configurations of the substituted and interstitial WSe2 at 0, 1, 2, 3, 4, and 5 ps are show in Fig. 2S and 3S.† For the substituted specimens (see Fig. 3), all the dopants still occupy the substituted Se site, except for the Br, Te, and I dopants, which slightly stand out from the surface of monolayer WSe2 due to their large dopant radius. For the interstitial specimens (see Fig. 4), all the dopants also keep in the interstitial site. However, some Se atoms in the B-, C-, Si-, S-, As-, Br-, Te-, or I-interstitial specimens also partly stand out from the surface of monolayer WSe2, which may be caused by their large dopant radius or unstable systems obtained based on the energy below.
The substituted energy Esub and interstitial energy Eint of all the doped monolayer WSe2 specimens were calculated by eqn (1) and the results are listed in Table 2, and where both W-rich and Se-rich conditions were considered for the substituted specimens. Under W-rich conditions, the O-, F-, S-, and Te-substituted specimens with negative Esub values were thermodynamically stable; otherwise, the others with positive Esub values were less stable than pristine monolayer WSe2. Under Se-rich conditions, the O- and S-substituted specimens with negative Esub values were still more stable than the pristine specimen, while the others with positive Esub values were less stable than pristine monolayer WSe2. This suggests that the preparation conditions have a great influence on the thermal stability of the substituted specimens; for instance, there are opposite Esub values for F- and Te-substituted specimens under W-rich and Se-rich conditions. Moreover, it was found that the NM dopants substitute preferentially for Se under W-rich conditions for all substituted specimens. The O-substituted monolayer WSe2 was the most stable due to its lowest Esub of −2.83 eV under W-rich conditions, while the O- and S-substituted monolayer WSe2 were more stable than the pristine specimen under both W-rich and Se-rich conditions. For the interstitial specimens, the F-, Cl-, and Br-interstitial specimens with negative Eint were thermodynamically stable, while the H-, N-, and I-interstitial specimens with small and positive Eint (0.80–1.57 eV) were thermodynamically metastable, and the others with large and positive Eint (beyond 2.37 eV) were thermodynamically unstable. Meanwhile, the substituted energies of H-, F-, Cl-, Br-, and I-doped specimens were higher than their interstitial energies, and thus, the H, F, Cl, Br, and I dopants are willing to locate at the interstitial site. Hence, we only discuss the more stable systems below.
 ) with the unit eV. The band gap Eg with unit eV. The energies of the Fermi level, the VBM, and the CBM (EF, EVBM, and ECBM) with the unit eV. The wavelengths of the optical absorption edges λedge with the unit nm, and the integral area A of the optical absorption curves in visible light region with uniform unit
) with the unit eV. The band gap Eg with unit eV. The energies of the Fermi level, the VBM, and the CBM (EF, EVBM, and ECBM) with the unit eV. The wavelengths of the optical absorption edges λedge with the unit nm, and the integral area A of the optical absorption curves in visible light region with uniform unit
| EW-richsub | ESe-richsub | Eint | EbondingW–NM | Eg | EF | EVBM | ECBM | λedge | A | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pristine | 0.00 | 0.00 | 0.00 | −5.19 | — | 1.75 | −3.20 | −3.20 | −1.45 | 705 | 1471 |
| H | 1.70 | 2.66 | 1.11 | — | −5.34 | 1.69 | −2.96 | −3.81 | −2.11 | 713 | 1461 |
| B | 2.36 | 3.32 | 5.64 | −6.25 | — | 1.80 | −3.56 | −3.84 | −2.04 | 718 | 1451 |
| C | 1.97 | 2.93 | 3.57 | −5.14 | — | 1.68 | −3.21 | −3.21 | −1.53 | 737 | 1554 |
| N | 0.35 | 1.31 | 1.57 | −6.22 | — | 1.79 | −3.40 | −3.71 | −1.92 | 728 | 1517 |
| O | −2.83 | −1.87 | 2.64 | −4.89 | — | 1.72 | −3.00 | −3.00 | −1.28 | 699 | 1576 |
| F | −0.55 | 0.41 | −1.80 | — | −5.43 | 1.71 | −3.73 | −3.83 | −2.12 | 722 | 1337 |
| Si | 1.80 | 2.76 | 5.04 | −4.98 | — | 1.68 | −3.18 | −3.18 | −1.50 | 737 | 1531 |
| P | 0.26 | 1.22 | 2.37 | −5.29 | — | 1.69 | −3.59 | −3.59 | −1.90 | 688 | 1411 |
| S | −1.24 | −0.28 | 8.53 | −5.07 | — | 1.74 | −3.14 | −3.14 | −1.40 | 690 | 1504 |
| Cl | 0.58 | 1.54 | −0.56 | — | −5.31 | 1.72 | −3.62 | −3.78 | −2.07 | 712 | 1399 |
| As | 0.72 | 1.68 | 4.57 | −5.47 | — | 1.67 | −3.75 | −3.75 | −2.08 | 690 | 1344 |
| Br | 1.04 | 2.00 | −0.46 | — | −5.54 | 1.77 | −3.75 | −3.88 | −2.11 | 722 | 1391 |
| Te | −0.74 | 0.22 | 6.96 | −4.59 | — | 1.72 | −3.04 | −3.04 | −1.32 | 700 | 1544 |
| I | 1.53 | 2.49 | 0.80 | — | −6.00 | 1.71 | −3.80 | −3.91 | −2.20 | 715 | 1354 |
Electronic performances
The calculated band structures of all the specimens are shown in Fig. 5, where the red dashed line at the zero point denotes the Fermi level (EF), while the positive and negative values indicate the conduction band (CB) and valance band (VB), respectively. For pristine monolayer WSe2, the Fermi level is locate at the valence band maximum (VBM) and the direct band gap (1.75 eV) is in good agreement with the experimental (1.60 eV) and other simulated (1.71 eV) results,43,44 albeit the experimental and simulated values are both affected by the preparation conditions and calculation methods.42 After introducing the NM dopant, the conduction band minimum (CBM), the VBM, and the band gap values of all the doped specimens were all changed. Compared with the Fermi level of each specimen itself, the VBM is still located at the Fermi level for the C-, O-, Si-, P-, S-, As-, and Te-doped specimens, but their CBM moves down to lower energy levels, and thus their band gap values are reduced slightly (less than 0.08 eV). On the other hand, although the CBM and VBM synchronously move down to the lower energy levels for the H-, B-, N-, F-, Cl-, Br-, and I-doped specimens, the variation trends of the band gap are different. For instance, the band gap values increase less than 0.05 eV for the B-, N-, and Br-doped specimens, but decrease less than 0.06 eV for the H-, F-, Cl-, and I-interstitial specimens. Although the band gap variation is quite small (less than 0.08 eV), the result is in agreement with others experimental value of S-doped monolayer WSe2,45 which will be explained below. Moreover, the impurity levels in the H-, C-, N-, F-, Si-, Cl-, Br-, and I-doped specimens lie above the EF, which can help to reduce the electronic transition energies of systems. Thus, the NM dopants affect the band gap values and electronic transition energies of systems.To gain a detailed insight into the influence of the dopants or chemical bond relaxations on the electronic performances of monolayer WSe2, the average PDOS value per W, Se, and NM atoms, for an equal and reasonable comparison, were calculated and are shown in Fig. 6. For pristine monolayer WSe2 (Fig. 6a), the W-5d states interact with the Se-4p states in both VB (−6.0–0.0 eV) and CB (1.2–2.5 eV), which are similar results to the other simulation results.42 After introducing NM dopants, the variation of the W-5d and Se-4p states, compared to pristine monolayer WSe2, are indistinctive, and thus the NM dopants have slight effects on the electronic structures of the W and Se host atoms. Otherwise, the W-5d states, respectively, interact with the Se-4p states in the W–Se bonds and the NM-p states in the newly formed W–NM bonds, while the Se-4p states interact with the NM-p states (or H-1s states) in the Se–NM bonds. However, the NM-p states (or H 1s states) are entirely different from each other, which means that the interaction intensities in the W–NM and Se–NM bonds are different. Moreover, an impurity level appears in some doped specimens; for instance, it is mainly made up of the W-5d states for the H-interstitial specimen, the C-2p, N-2p, and Si-2p states, respectively, for the C-, N-, and Si-substituted specimens, and the F-2p, Cl-3p, Br-4p, and I-5p states for the F-, Cl-, Br-, and I-interstitial specimens. Briefly, the electronic structures of the W–NM and Se–NM bonds are decided by the NM dopants, and the W–Se bonds of monolayer WSe2 are broadly the same before and after doping NM atoms. Thus, the electronic performances of the doped specimens are decided by the newly formed W–NM or Se–NM bonds.
Further, the electron density differences of all specimens were calculated and are exhibited in Fig. 7, where the red and blue regions indicate the electron accumulation and loss, respectively. The red areas around the Se atom and the blue regions around the W atom represent the ionic bonds between W and Se atoms in pristine monolayer WSe2 (Fig. 7a). Moreover, the red areas also appear in the middle of W and Se atoms, which indicate the covalent bonding between them. After introducing a NM atom into the monolayer WSe2, the electronic distributions around Se3 and Se4 atoms adding W–Se3 and W–Se4 bonds were almost invariant, while the electronic distributions around Se2 and W–Se2 bonds were altered slightly; however, the electronic distributions around Se1 or NM atoms adding W–Se1 or W–NM bonds were altered obviously. This means that the NM dopants only affected the electronic structures of the neighboring W and Se1 or Se2 atoms, and thus the band gap values of the doped specimens were only affected by the newly formed W–NM or Se–NM bonds. Meanwhile, the electronic distributions around the NM dopants and W–NM (or Se–NM) bonds were totally different from pristine monolayer WSe2, while the color depth of W, Se, and NM atoms adding the W–NM and Se–NM bonds were different for each doped specimen (Fig. 7b–o). This means that the electronic performances of the doped specimens are mostly altered by the NM dopants and W–NM (or Se–NM) bonds. Thus, the bonding strength of W–NM or Se–NM bonds should be determined by NM dopants.
Optical and catalytic parameters
The optical absorption curves of all the considered specimens were calculated and are shown in Fig. 8. It was found that the wavelength of the optical absorption edge (λedge) was near 705 nm for pristine monolayer WSe2 (Fig. 8a). After introducing the NM dopants, the optical absorption curves of specimens were changed. For instance, compared to pristine WSe2, the optical absorption edges are red-shifted less than 32 nm for the H-, B-, C-, N-, F-, Si-, Cl-, Br-, and I-doped specimens but are blue-shifted less than 17 nm for the others (see Table 2). Meanwhile, the integrated area (A) of the optical absorption curves in the visible light regions are also listed in Table 2, where the results indicate that the A values reduce less than 8% for the H-, B-, F-, P-, Cl-, As-, Br-, and I-doped specimens, but increase less than 9.5% for the others. In a word, the NM dopants can affect the optical performance of monolayer WSe2.The active sites of all the specimens were defined by the HOMO and LUMO levels (see Fig. 9). For pristine monolayer WSe2, the HOMO was made up of W-5d orbits, while the LUMO was mainly composed of W-5d and Se-4p orbits, and thus the excited electrons and holes occurring on the neighboring W and Se atoms can be recombined easily, which reduces the photocatalytic active. However, the HOMO and LUMO are changed by the NM dopants, whereby the HOMO mainly locates in the dopant and its surrounding atoms, while the LUMO is far away from the dopants for the F-, P-, Cl-, As-, Br-, and I-doped specimens, and the H-doped specimen show the opposite trends, and in which the visible separation of HOMO and LUMO contributes to the separation of photogenerated e−/h+ pairs. Moreover, the HOMO and LUMO were only partly separated after substituting one Se atom by one N, O, or S atom, which may slightly enhance the separation of photogenerated e−/h+ pairs. However, both the HOMO and LUMO mainly locate in the dopant and its surrounding atoms for the B-, C-, Si-, and Te-doped specimens, and thus the dopants will become the recombination centers for photogenerated e−/h+ pairs. In brief, the H-, F-, P-, Cl-, As-, Br-, and I-doped specimens could have a high photocatalytic efficiency due to the separation of HOMO and LUMO.
 | ||
| Fig. 9 HOMO (pink areas) and LUMO (yellow areas) of pristine and NM-doped monolayer WSe2. The isosurface is taken at a value of 0.003 e Bohr−3. | ||
Further, the adjustment of the redox potential by the dopants was studied by experiments and by using calculation methods,46–48 and the energies of the Fermi level, the CBM, and the VBM (EF, ECBM, and EVBM) of all the specimens, compared with the vacuum level, are listed in Table 2 and shown in Fig. 10. It was found the ECBM and EVBM values are affected by the number of free electrons of the dopants, which denote the redox potentials of the specimens. For instance, the NM atoms with odd free electrons (one free electron for H, F Cl, Br, or I atoms, three free electrons for N, P, or As atoms, and five free electrons for the B atom) obviously reduce the reduction potential by 0.39–0.71 eV and enhance the oxidation potential by 0.45–0.75 eV. The reduced reduction potential and enhanced oxidation potential may be caused by the lone electrons in the NM atoms with one, three, or five free electrons. The one lone electron in the NM atoms can also interact with the W or Se1 atom to enhance the interaction intensity of the W–NM or W–Se2 bonds. The bonding energy of the W–NM or W–Se2 bonds (EbondingW–NM and  ) for the H-, F-, Cl-, Br-, I-, N-, P-, As-, and B-doped specimens were lower than that of pristine specimen (see Table 2), which displays its stronger interaction intensity. On the other hand, the O, S, and Te atoms with two free electrons enhance the reduction potential and reduce the oxidation potential, while the Si and C atoms with four free electrons have only slight effects on the redox potentials of monolayer WSe2. Thus, this demonstrates that we can adjust the redox potentials of monolayer WSe2 by introducing different kinds of NM atoms for various catalytic reactions.
) for the H-, F-, Cl-, Br-, I-, N-, P-, As-, and B-doped specimens were lower than that of pristine specimen (see Table 2), which displays its stronger interaction intensity. On the other hand, the O, S, and Te atoms with two free electrons enhance the reduction potential and reduce the oxidation potential, while the Si and C atoms with four free electrons have only slight effects on the redox potentials of monolayer WSe2. Thus, this demonstrates that we can adjust the redox potentials of monolayer WSe2 by introducing different kinds of NM atoms for various catalytic reactions.
 | ||
| Fig. 10 ECBM (a) and EVBM (b) of pristine and NM-doped monolayer WSe2, as distinguished by the number of free electrons of the dopants. | ||
Conclusions
In summary, a series of NM-doped monolayer WSe2 was studied by first-principles calculations and the effects of the dopants on the structural, thermal, electronic, optical, and catalytic performances were investigated in detail. In terms of the structural and thermal aspects, the O-doped monolayer WSe2 was the most stable due to its lowest energy under W-rich conditions, while the NM dopants substituted preferentially for Se under W-rich conditions. In terms of the electronic performances, the dopants affect the electronic structures with the newly formed W–NM or Se–NM bonds, and the bonding strength of the W–NM or Se–NM bonds. In terms of the optical aspect, the optical absorption edges are red-shifted or blue-shifted less than 32 nm and the optical absorption intensities are enhanced or reduced less than 9.5% for all the doped specimens. In terms of the photocatalytic active aspect, the H, F, P, Cl, As, Br, and I dopants contributed to the separation of the HOMO and LUMO, but the B, C, Si, and Te dopants became recombination centers for the photogenerated e−/h+ pairs. In terms of the redox ability aspect, the NM atoms with odd free electrons obviously reduced the reduction potential by 0.39–0.71 eV and enhanced the oxidation potential by 0.45–0.75 eV. Thus, we demonstrated that we can adjust the redox potentials of monolayer WSe2 by introducing different kinds of NM atoms for various catalytic reactions and that the H-, F-, P-, Cl-, As-, Br-, and I-doped specimens have excellent photocatalysis capability.Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (No. 51402274, 21401180 and 51402277), the Science and Technology of Zhejiang Province for Returned Researcher and the Special Program for Applied Research on Super Computation of the NSFC-Guangdong Joint Fund (the second phase). Computational resources were provided by the Jilin University.References
- Z. Yin, H. Li, H. Li, L. Jiang, Y. Shi, Y. Sun, G. Lu, Q. Zhang, X. Chen and H. Zhang, ACS Nano, 2012, 6, 74–80 CrossRef CAS PubMed.
- H. S. Liu, N. N. Han and J. J. Zhao, RSC Adv., 2015, 5, 17572–17581 RSC.
- N. Latiff, W. Z. Teo, Z. Sofer, S. Huber, A. C. Fisher and M. Pumera, RSC Adv., 2015, 5, 67485–67492 RSC.
- P. P. Hankare, A. H. Manikshete, D. J. Sathe, P. A. Chate, A. A. Patil and K. M. Garadkar, J. Alloys Compd., 2009, 479, 657 CrossRef CAS.
- Z. Ni, H. Zhong, X. Jiang, R. Quhe, G. Luo, Y. Wang, M. Ye, J. Yang, J. Shi and J. Lu, Nanoscale, 2014, 6, 7609–7618 RSC.
- A. Kumar and P. K. Ahluwalia, J. Alloys Compd., 2014, 587, 459–467 CrossRef CAS.
- M. Kertesz and R. Hoffmann, J. Am. Chem. Soc., 1984, 106, 3453–3460 CrossRef CAS.
- G. L. Hao, L. Z. Kou, D. L. Lu, J. Peng, J. Li, C. Tang and J. X. Zhong, J. Appl. Phys., 2016, 119, 035301 CrossRef.
- N. Flöry, A. Jain, P. Bharadwaj, M. Parzefall, T. Taniguchi, K. Watanabe and L. Novotny, Appl. Phys. Lett., 2015, 107, 2123106 CrossRef.
- B. Wang, S. M. Eichfield, D. Wang, J. A. Robinson and M. A. Haque, Nanoscale, 2015, 7, 14489–14495 RSC.
- J. H. Guo, Y. T. Shi, X. G. Bai, X. C. Wang and T. L. Ma, J. Mater. Chem. A, 2015, 3, 24397–24404 CAS.
- W. Liu, J. Kang, D. Sarkar, Y. Khatami, D. Jena and K. Banerjee, Nano Lett., 2013, 13, 1983 CrossRef CAS PubMed.
- K. Gong, L. Zhang, D. P. Liu, L. Liu, Y. Zhu, Y. H. Zhao and H. Guo, Nanotechnology, 2014, 25, 43 Search PubMed.
- A. Y. Gao, E. F. Liu, M. S. Long, W. Zhou, Y. Y. Wang, T. L. Xia, W. D. Hu, B. G. Wang and F. Mao, Appl. Phys. Lett., 2016, 108, 223501 CrossRef.
- J. R. Mckone, R. A. Potash, F. J. Disalvo and H. D. Abruna, Phys. Chem. Chem. Phys., 2015, 17, 13984–13991 RSC.
- X. Y. Yu, M. S. Prévot, N. Guijarro and K. Sivula, Nat. Commun., 2015, 6, 7596 CrossRef PubMed.
- B. Amin, T. P. Kaloni and U. Schwingenschlogl, RSC Adv., 2014, 4, 34561–34565 RSC.
- S. F. Wang, W. J. Zhao, F. Giustiniano and G. Eda, Phys. Chem. Chem. Phys., 2016, 18, 4304–4309 RSC.
- P. D. Zhao, D. Kiriya, A. Azcatl, C. X. Zhang, M. Tosun, Y.-S. Liu, M. Hettick, J. S. Kang, S. McDonnell, J. H. Guo, K. Cho, R. M. Wallace and A. Javey, ACS Nano, 2014, 8(10), 10808–10814 CrossRef CAS PubMed.
- Y. J. Zheng, Y. L. Huang, Y. F. Cheng, W. J. Zhao, G. Eda, C. D. Spataru, W. J. Zhang, Y.-H. Chang, L.-J. Li, D. Z. Chi, S. Y. Quek and A. T. S. Wee, ACS Nano, 2016, 10(2), 2476–2484 CrossRef CAS PubMed.
- Y. Guo, D. Liu and J. Robertson, Appl. Phys. Lett., 2015, 106, 173106 CrossRef.
- S. J. Wi, M. K. Chen, D. Li, H. Nam, E. Meyhofer and X. G. Liang, Appl. Phys. Lett., 2015, 107, 062102 CrossRef.
- C.-H. Chen, C.-L. Wu, J. Pu, M.-H. Chiu, P. Kumar, T. Takenobu and L.-J. Li, 2D materials., 2014, 1, 3 CrossRef.
- M. Kriener, A. Kikkawa, T. Suzuki, R. Akashi, R. Arita, Y. Tokura and Y. Taguchi, Phys. Rev. B: Condens. Matter Mater. Phys., 2015, 91, 075205 CrossRef.
- A. Allain and A. Kis, ACS Nano, 2014, 8(7), 7180–7185 CrossRef CAS PubMed.
- Y. Zhang, M. M. Ugeda, C. H. Jin, S. F. Shi, A. J. Bradley, H. Ryu, S. J. Tang, B. Zhou, F. Wang, M. F. Crommie, Z. Hussain, Z. X. Shen and S. K. Mo, Nano Lett., 2016, 16(4), 2485–2491 CrossRef CAS PubMed.
- J. J. Zhu and U. Schwingenschlögl, J. Mater. Chem. C, 2015, 3, 3946 RSC.
- G. J. Gil, A. Pham, A. B. Yu and S. Li, J. Phys.: Condens. Matter, 2014, 26, 30 CrossRef PubMed.
- A. A. Mitioglu, P. Plochocka, A. Granados del Aguila, P. C. M. Christianen, G. Deligeorgis, S. Anghel, L. Kulyuk and D. K. Maude, Nano Lett., 2015, 15(7), 4387–4392 CrossRef CAS PubMed.
- H. T. Wang, D. S. Kong, P. Johanes, J. J. Cha, G. Y. Zheng, K. Yan, N. Liu and Y. Cui, Nano Lett., 2013, 13(7), 3426–3433 CrossRef CAS PubMed.
- S. Y. Hu, M. C. Cheng, K. K. Tiong and Y. S. Huang, J. Phys.: Condens. Matter, 2005, 17, 23 CrossRef.
- J. Huang, W. H. Wang, Q. Fu, L. Yang, K. Zhang, J. Y. Zhang and B. Xiang, Nanotechnology, 2016, 27, 23 Search PubMed.
- M. L. Zou, J. D. Chen, L. F. Xiao, H. Zhu, T. T. Yang, M. Zhang and M. L. Du, J. Mater. Chem. A, 2015, 3, 18090–18097 CAS.
- Y. F. Zhao, C. Li, Y. Y. Gong, T. Wang and C. Q. Sun, Phys. Chem. Chem. Phys., 2014, 16, 21446 RSC.
- M. D. Segall, P. J. D. Lindan, M. J. Probert, C. J. Pickard, P. J. Hasnip, S. J. Clark and M. C. Payne, J. Phys.: Condens. Matter, 2002, 14, 2717 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- D. H. Hamalm, M. Schluter and C. Chiang, Phys. Rev. Lett., 1979, 43, 1494 CrossRef.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558 CrossRef CAS.
- C. Su, H. Jiang and J. Feng, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 87, 075453 CrossRef.
- A. H. Reshak and S. Auluck, Phys. Rev. B: Condens. Matter Mater. Phys., 2003, 68, 195107 CrossRef.
- N. D. Boscher, C. J. Carmalt and I. P. Parkin, J. Mater. Chem., 2006, 16, 122 RSC.
- L.-P. Feng, J. Su and Z.-T. Liu, J. Alloys Compd., 2015, 639, 210–214 CrossRef CAS.
- A. Kumara and P. K. Ahluwalia, Eur. Phys. J. B, 2012, 85, 186 CrossRef.
- H. Zeng, G.-B. Liu, J. Dai, Y. Yan, B. Zhu, R. He, L. Xie, S. Xu, X. Chen, W. Yao and X. Cui, Sci. Rep., 2013, 3, 1608 Search PubMed.
- J. Huang, W. H. Wang, Q. Fu, L. Yang, K. Zhang, J. Y. Zhang and B. Xiang, Nanotechnology, 2016, 27, 13 Search PubMed.
- T. Shiraishi, G. Juhász, T. Shiraki, N. Akizuki, Y. Miyauchi, K. Matsyda and N. Nakashima, J. Phys. Chem. C, 2016, 120(29), 15632 CAS.
- S. Lu, C. Li, Y. F. Zhao, Y. Y. Gong, L. Y. Niu and X. J. Liu, Appl. Surf. Sci., 2016, 384, 360–367 CrossRef CAS.
- S. Lu, Z. W. Chen, C. Li, H. H. Li, Y. F. Zhao, Y. Y. Gong, L. Y. Niu, X. J. Liu, T. Wang and C. Q. Sun, J. Mater. Chem. A, 2016, 4, 14827–14838 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra15697e |
| This journal is © The Royal Society of Chemistry 2016 |