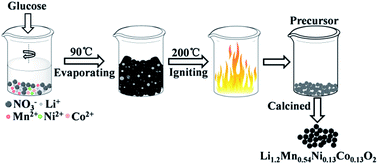
Lithium-rich layered Li1.2Ni0.13Co0.13Mn0.54O2 cathode materials have been successfully fabricated by a glucose-assisted combustion method combined with a calcination treatment. The effect of the amount of glucose fuel on the properties of the prepared materials is investigated by X-ray diffraction, scanning electron microscopy, X-ray photoelectron spectroscopy and electrochemical measurements. The results show that the nano-sized cathode material obtained at a fuel ratio of φ = 1 exhibits uniform fine well-crystallized particles with the largest specific surface area, leading to excellent cyclic capability and rate performance. It delivers the highest initial discharge capacity of 280.5 mA h g−1 with a capacity retention of 84% after 50 cycles at 0.1C (25 mA g−1). Besides, after cycling at an increasing rate from 0.2C to 3C, the electrode retained 90.3% (230.2 mA h g−1) of the initial discharge capacity when the rate was recovered back to 0.2C.