Coaxial ultrathin Co1−yFeyOx nanosheet coating on carbon nanotubes for water oxidation with excellent activity†
Abstract
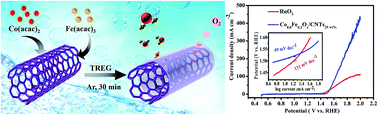
The rational design of high-performance and non-precious metal electrocatalysts is highly important for energy technologies. Herein, for the first time, a coaxial ultrathin Co1−yFeyOx nanosheet coating on carbon nanotubes (denoted as Co1−yFeyOx/CNTs) is prepared by a one-step pyrolysis method. The fabrication procedure is ultrafast and uncomplicated, furthermore, it does not need any reducing agent, alkali, or surfactant. In 1.0 M KOH, the obtained optimal hybrids Co0.8Fe0.2Ox/CNTs25 wt% catalyze oxygen evolution reactions (OER) with a very sharp onset potential (∼1.45 V) and an exceptional over-potential (0.28 V, at 10 mA cm−2) for more than 14 h, benefiting from the abundant active sites of ultrathin Co0.8Fe0.2Ox nanosheets, the hierarchical tubular architecture, and the strong cooperative effect among Co, Fe and CNTs. Remarkably, the excellent activity and durability of Co0.8Fe0.2Ox/CNTs25 wt% for the OER is superior to commercial RuO2 and many other highly active precious-metal/transition-metal catalysts reported to date. The design concept for tubular iron-group binary metal nanosheet hybrids creates new pathways for energy technologies.

 Please wait while we load your content...
Please wait while we load your content...