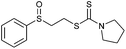
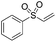
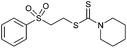
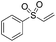
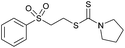
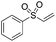
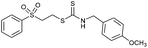
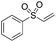
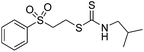
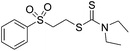
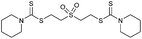
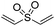
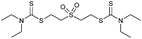
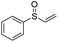
Multicomponent synthesis of dithiocarbamates starting from vinyl sulfones/sulfoxides and their use in polymerization reactions†
Azim Ziyaei Halimehjani*a,
Reza Mohtashama,
Abbas Shockravia and
Jürgen Martensb
aFaculty of Chemistry, Kharazmi University, P. O. Box 15719-14911, 49 Mofateh St., Tehran, Iran. E-mail: ziyaei@khu.ac.ir; Fax: +98 21 88820992; Tel: +98 21 88848949
bInstitut für Chemie, Carl von Ossietzky Universität Oldenburg, P. O. Box 2503, Carl-von-Ossietzky-Str. 9–11, 26111 Oldenburg, Germany
First published on 3rd August 2016
Abstract
A catalyst- and solvent-free procedure for the synthesis of dithiocarbamates from amines, carbon disulfide (CS2) and vinyl sulfones/sulfoxides is reported. This protocol provides a simple, high yielding and green approach for the synthesis of novel dithiocarbamates with commercially available starting materials. Also, three-component polyaddition of diamines, CS2 and divinyl sulfone was successfully performed without the use of a catalyst to provide the corresponding polymers containing dithiocarbamate (dithiourethane) and sulfone moieties.
Multicomponent reactions (MCRs) provide an efficient route for the rapid synthesis of complex molecules with often biologically relevant scaffold structures. Therefore, the design of novel MCRs or modification of the current MCRs with green procedures have attracted great attention from research groups working in various areas such as drug discovery, synthetic organic chemistry, polymer chemistry and materials science.1 Performing organic transformations in solvent-free conditions is in agreement with the goals of green chemistry due to the obvious reduction of pollution, decreasing handling and labor costs, simplification of experimental procedures and work up techniques which would be especially important during industrial production.2
Dithiocarbamates (DTCs) are a well-known category of organic compounds with diverse applications in synthetic organic chemistry as intermediates,3 as fungicides and pesticides in agriculture,4 sulfur vulcanization in rubber manufacturing,5 radical chain transfer agents in the reversible addition fragmentation chain transfer (RAFT) polymerizations,6 and medicinal chemistry.7 Also, due to the high affinity of the sulfurs in DTC group for coordination with metals, these compounds have found wide applications as an adsorbent for the recovery, extraction and determination of metals such as gold, silver, lead, mercury, cadmium, copper, and etc.8 Among them, natural and synthetic polymers bearing DTC moieties in their structure are well-documented.9 Polymers containing dithiocarbamate groups usually possess inherently greater stability to hydrolytic breakdown compared to xanthates, which makes them as suitable candidates for heavy metal cations removal from waste and drinking water.10 Most of the synthesized or modified naturally abundant polymers contain the dithiocarbamate groups as side chain. For the synthesis of polymers containing the dithiocarbamate group in their backbone, recently, a three-component polyaddition of diamines, CS2 and diacrylates in water was reported by Nagai et al. to produce the corresponding poly(dithiourethane-amine)s for the selective adsorption of Au(III) under acidic conditions.11
Reactions of amines with CS2 provide the dithiocarbamic acids as intermediates which are good nucleophiles and react with various electrophiles such as alkyl halides,12 epoxides,13 carbonyls,14 electron-riched alkenes,15 α,β-unsaturated carbonyl compounds,16 etc.17 Although Michael addition of dithiocarbamic acid salts to electron-deficient alkenes in the presence of a base or acid as catalyst and using organic solvents have been well documented,18 there are only few reports on the one-pot three-component synthesis of these compounds under catalyst-free conditions.16 Here, we report a simple and green protocol for the synthesis of DTCs via MCR of amines, CS2 and vinyl sulfones/sulfoxides. Also, this protocol was extended for the synthesis of polymers using diamines, CS2 and divinyl sulfone.
In continuation of our research toward the development of green organic chemistry by performing organic reactions under solvent-free conditions and our experiences on the synthesis of DTCs using α,β-unsaturated carbonyl compounds,16a herein we report an efficient, and environmentally benign procedure for the Michael addition reaction of in situ prepared dithiocarbamic acid to vinyl sulfones and sulfoxides under solvent- and catalyst-free conditions at room temperature (Scheme 1).
 | ||
| Scheme 1 Michael addition reaction of in situ prepared dithiocarbamic acid to vinyl sulfones and sulfoxides. | ||
A simple mixing of amines, CS2 and vinyl sulfones/sulfoxides produced the corresponding Michael adducts in high to excellent yield. This three components reaction is completed in 6 h at room temperature without using any catalyst. Primary and secondary amines are suitable substrates in this protocol. Aromatic amines were applied in this reaction with no result. Vinyl sulfoxide was applied in this protocol as well as vinyl sulfone. By using divinyl sulfone in this protocol, the corresponding bis DTCs were achieved in excellent yields via a one-pot pseudo-five-component reaction. The structures of products were elucidated using IR, 1H and 13C NMR and HRMS analyses.
After successful Michael addition reaction of dithiocarbamic acids derived from monoamines to divinyl sulfone, we envisaged that this protocol can be expanded to diamines for the synthesis of polymers (Scheme 2). For this purpose, the three-component polyaddition of 1,3-propanediamine, CS2, and divinyl sulfone was investigated under similar conditions described by Nagai et al.11 We observed that low yield of polymer was obtained. The low yield in water may be attributed to the low solubility of the diamine–CS2 adducts in water which makes stirring difficult. Also, under this condition, direct Michael addition reaction of amines to divinyl sulfone was observed in the product. To increase the reaction yield, we repeated the similar reaction in various organic solvents. We observed that the best yield (90%) was obtained when a mixture of ethanol and DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was applied. The low yield in other organic solvents can also be attributed to the low solubility of the intermediate, bisdithiocarbamic acid, and its precipitation in the reaction mixture which avoid further reacting with divinyl sulfone. Under optimized reaction conditions (see general procedure in Experimental section), polymers P1–P8 were synthesized and the results are summarized in Scheme 2. The incorporation ratio of CS2 for all products was determined more than 95% (due to detection limit of NMR instrument) by the integration ratio of dithiocarbamate adducts and direct amine adducts.
1) was applied. The low yield in other organic solvents can also be attributed to the low solubility of the intermediate, bisdithiocarbamic acid, and its precipitation in the reaction mixture which avoid further reacting with divinyl sulfone. Under optimized reaction conditions (see general procedure in Experimental section), polymers P1–P8 were synthesized and the results are summarized in Scheme 2. The incorporation ratio of CS2 for all products was determined more than 95% (due to detection limit of NMR instrument) by the integration ratio of dithiocarbamate adducts and direct amine adducts.
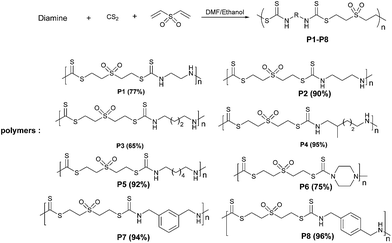 | ||
| Scheme 2 Three-component polyaddition of diamine, CS2, and divinyl sulfone for the synthesis of polymers P1–P8. | ||
IR spectroscopic analysis of the obtained polymers indicated the presence of a broad band at 3200–3300 cm−1 for the N–H groups, two strong bands at 1100–1150 and 1300–1350 cm−1 for the symmetrical and asymmetrical stretching bands of SO2 group, respectively, and a strong band at 1500–1550 for the thiocarbonyl of dithiocarbamate group. The 1H NMR spectra of the polymers exactly confirms the thia-Michael addition products. The methylenes protons of CH2SO2, CH2S and CH2NH groups were observed between 3.5 and 5.0 ppm, while for the direct addition of amines to divinyl sulfone, usually the chemical shift of CH2N protons do not exceed 3.5 ppm. Also, the N–H proton was assigned between 10.0 and 10.5 ppm, which exactly approve the presence of dithiocarbamate moieties in the structures (Table 1).
The inherent viscosity of the polymers was measured at a concentration of 0.5 g dL−1 in DMSO using Cannon-Fenske viscometer at 30 °C. As shown in Table 2, the obtained polymers had inherent viscosities in the range of 0.29–0.72 dL g−1.
| Polymer | ηinha (dL g−1) | T10 (°C) | Tmax (°C) | Tg (°C) | Solubility in various solventsb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DMSO | DMF | DMAc | THF | CH3OH | H2O | CHCl3 | |||||
| a Measured at a polymer concentration of 0.5 g dL−1 in DMSO at 30 °C.b The qualitative solubility was tested with 5 mg of a sample in 1 mL of stirred solvent. Notation: ++, soluble at room temperature; +, soluble at 100 °C; ±, partially soluble; (−), insoluble. DMSO, dimethyl sulfoxide; DMF, N,N-dimethylformamide; DMAc, N,N-dimethylacetamide; THF, tetrahydrofuran. | |||||||||||
| P1 | 0.24 | ++ | + | + | ± | − | − | − | |||
| P2 | 0.26 | 214 | 252 | 76 | ++ | ++ | ++ | + | − | − | − |
| P3 | 0.30 | 185 | 258 | 73 | ++ | ++ | ++ | + | − | − | − |
| P4 | 0.47 | ++ | ++ | ++ | ++ | − | − | − | |||
| P5 | 0.62 | ++ | ++ | ++ | ++ | − | − | − | |||
| P6 | 0.37 | 228 | 270 | 149 | + | ± | ± | − | − | − | − |
| P7 | 0.56 | ++ | ++ | ++ | ++ | − | − | − | |||
| P8 | 0.61 | ++ | ++ | ++ | ± | − | − | − | |||
The solubility behavior of polymers was tested qualitatively in several organic solvents at 5% (w/v), and the results are also included in Table 2. Most of the polymers were soluble in polar aprotic solvents such as DMSO, DMF and DMAc at room temperature. Polymer P1 was dissolved in DMAc and DMF at 100 °C. Polymer P6 was soluble in DMSO at 100 °C and slightly soluble in DMF and DMAc. Polymers P4, P5 and P7 were soluble in THF at room temperature which can be attributed to the long chain aliphatic groups and lower polarity of polymers.
The polymers were insoluble in methanol and chloroform.
The glass transition temperature (Tg), T10 and Tmax of the selected polymers are also summarized in Table 2. The lower Tg of the polymer P3 compare to the polymer P2 can be attributed to the increase in the methylene unit of the diamine, suggesting increasing the mobility of the main chain. Also the piperazine based polymer P6 showed higher Tg compare to polymers P2 and P3 due to its rigid structure. Also, from TGA results of the selected polymers, it can be concluded that the polymer P6 showed higher thermal stability compared to other polymers.
Polymer P5 was considered for GPC analysis due to its high solubility in THF. The number-average (Mn) and weight-average (Mw) molecular weights of polymer P5 was determined as 6070 and 2633, respectively, with molecular weight distribution (poly dispersity index) (PDI) of 2.30, which is mostly superposable with the oligomer structure compare to considering “polymer” word for such compounds.
Conclusions
In conclusion, a catalyst- and solvent-free procedure for the synthesis of DTCs from amines, carbon disulfide (CS2) and vinyl sulfones/sulfoxides is reported. The main advantages of this protocol are simplicity, high yielding and green approach for the synthesis of novel DTCs with commercially available starting materials. Also, this protocol was applied for three-component polyaddition of diamines, CS2 and divinyl sulfone for synthesis of the corresponding polymers containing dithiocarbamate (dithiourethane) and sulfone moieties.Acknowledgements
We are grateful to the Faculty of Chemistry of Kharazmi University for supporting this work.Notes and references
- (a) S. Kobayashi, Chem. Soc. Rev., 1999, 28, 1 RSC; (b) D. Tejedor, D. Gonzalez-Cruz, A. Santos-Exposito, J. J. Marrero-Tellado, P. de Armas and F. Garcyaa-Tellado, Chem.–Eur. J., 2005, 11, 3502 CrossRef CAS PubMed; (c) J. Zhu, Eur. J. Org. Chem., 2003, 1133 CrossRef CAS and references therein.
- (a) M. A. P. Martins, C. P. Frizzo, D. N. Moreira, L. Buriol and P. Machado, Chem. Rev., 2009, 109, 4140 CrossRef CAS PubMed; (b) M. Li, H. Cao, Y. Wang, X.-L. Lv and L.-R. Wen, Org. Lett., 2012, 14, 3470 CrossRef CAS PubMed; (c) J. F. Collados, E. Toledano, D. Guijarro and M. Yus, J. Org. Chem., 2012, 77, 5744 CrossRef CAS PubMed.
- (a) R. Wong and S. J. Dolman, J. Org. Chem., 2007, 72, 3969 CrossRef CAS PubMed; (b) A. Ziyaei Halimehjani, H. Maleki and M. R. Saidi, Tetrahedron Lett., 2009, 50, 2747 CrossRef; (c) L. Jamir, U. B. Sinha, J. Nath and B. K. Patel, Synth. Commun., 2012, 42, 951 CrossRef CAS; (d) A. Ziyaei Halimehjani, L. Hasani, M. A. Alaei and M. R. Saidi, Tetrahedron Lett., 2016, 57, 883 CrossRef CAS , and references therein.
- (a) S. Kanchi, P. Singh and K. Bisetty, Arabian J. Chem., 2014, 7, 11 CrossRef CAS; (b) M. A. Hussein, A. N. El-Shorbagi and A. R. Khallil, Arch. Pharm., 2001, 334, 305 CrossRef CAS; (c) G. Eng, X. Song, Q. Duong, D. Strickman, J. Glass and L. May, Appl. Organomet. Chem., 2003, 17, 218 CrossRef CAS.
- (a) P. J. Nieuwenhuizen, A. W. Ehlers, J. G. Haasnoot, S. R. Janse, J. Reedijk and E. J. Baerends, J. Am. Chem. Soc., 1999, 121, 163 CrossRef CAS; (b) W. E. Messer, British Patent 496560, 1930; Chem. Abstr., 1939, 33, 4080.
- (a) J. T. Lai and R. Shea, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 4298 CrossRef CAS; (b) A. Dureaault, Y. Gnanou, D. Taton, M. Destarac and F. Leising, Angew. Chem., Int. Ed., 2003, 42, 2869 CrossRef PubMed; (c) M. Bathfield, F. D'Agosto, R. Spitz, M. T. Charreyre and T. Delair, J. Am. Chem. Soc., 2006, 128, 2546 CrossRef CAS PubMed.
- (a) S. T. V. S. Kiran Kumar, L. Kumar, V. L. Sharma, A. Jain, R. K. Jain, J. P. Maikhuri, M. Kumar, P. K. Shukla and G. Gupta, J. Med. Chem., 2008, 43, 2247 CrossRef CAS PubMed; (b) L. Kumar, N. Lal, V. Kumar, A. Sarswat, S. Jangir, V. Bala, L. Kumar, B. Kushwaha, A. K. Pandey, M. I. Siddiqi, P. K. Shukla, J. P. Maikhuri, G. Gupta and V. L. Sharma, Eur. J. Med. Chem., 2013, 70, 68 CrossRef CAS PubMed; (c) R. P. Tripathi, A. R. Khan, B. S. Setty and A. P. Bhaduri, Acta Pharm., 1996, 46, 169 CAS; (d) O. Ates, A. Kocabalkanli, N. Cesur and G. Otuk, Il Farmaco, 1998, 53, 541 CrossRef CAS PubMed.
- (a) T. H. Zhang, X. Q. Shan, R. X. Liu, H. X. Tang and S. Z. Zhang, Anal. Chem., 1998, 70, 3964 CrossRef CAS; (b) J. M. Lo and J. D. Lee, Anal. Chem., 1994, 66, 1242 CrossRef CAS; (c) R. S. Praveen, G. R. K. Naidu and T. P. Rao, Anal. Chim. Acta, 2007, 600, 205 CrossRef CAS PubMed.
- (a) A. Khan, S. Badshah and C. Airoldi, Colloids Surf., B, 2011, 87, 88 CrossRef CAS PubMed; (b) K. A. Venkatesan, T. G. Srinivasan and P. R. V. Rao, Colloids Surf., A, 2001, 180, 277 CrossRef CAS; (c) M. E. Mahmoud, Anal. Chim. Acta, 1999, 398, 297 CrossRef CAS; (d) C. Van Kerckhoven, H. Van den Broeck, G. Smets and J. Huybrechts, Makromol. Chem., 1991, 192, 101 CrossRef CAS; (e) M. Lieder and C. W. Schapfer, J. Appl. Electrochem., 2001, 31, 1321 CrossRef CAS.
- C. K. Wagner, G. Hall, B. Riegel, J. DE Virgilio, V. Kamath and G. Germann, J. Appl. Polym. Sci., 1986, 31, 1797 CrossRef CAS.
- D. Nagai, T. Imazki, H. Morinaga, H. Oku and K. I. Kasuya, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 845 CrossRef CAS.
- N. Azizi, F. Aryanasab and M. R. Saidi, Org. Lett., 2006, 8, 5275 CrossRef CAS PubMed.
- (a) A. Ziyaei Halimehjani and M. R. Saidi, Can. J. Chem., 2006, 84, 1515 CrossRef; (b) N. Azizi, B. Pourhasan, F. Aryanasab and M. R. Saidi, Synlett, 2007, 2797 CrossRef CAS.
- A. Ziyaei Halimehjani, M. Hajiloo Shayegan, M. M. Hashemi and B. Notash, Org. Lett., 2012, 14, 3838 CrossRef CAS PubMed.
- A. Ziyaei Halimehjani, K. Marjani and A. Ashouri, Green Chem., 2010, 12, 1306 RSC.
- (a) N. Azizi, F. Aryanasab, L. Torkiyan, A. Ziyaei and M. R. Saidi, J. Org. Chem., 2006, 71, 3634 CrossRef CAS PubMed; (b) G. Shengrong, Y. Yanqin and Z. Chunniu, Youji Huaxue, 2012, 32, 907 CrossRef; (c) N. Kaiwei and Z. Peijun, Guangdong Huagong, 2012, 39, 307 Search PubMed.
- (a) F. C. Brown, Chem. Rev., 1961, 61, 463 CrossRef CAS; (b) S. P. Singh, S. S. Parmar, K. V. Raman and I. Stenberg, Chem. Rev., 1981, 81, 175 CrossRef CAS; (c) S. Moghimi, M. M. Heravi, H. A. Oskooie and Y. S. Beheshtiha, Sci. Iran., Trans. C, 2014, 21, 748 CAS; (d) N. Azizi, M. Hasani, M. Khajeh and M. Edrisi, Tetrahedron Lett., 2015, 56, 1189 CrossRef CAS; (e) I. Yavari, R. Hajinasiri, A. Z. Sayyed-Alangi and N. Iravani, Monatsh. Chem., 2008, 139, 1029 CrossRef CAS; (f) A. Alizadeh, S. Rostamnia, N. Zohreh and R. Hosseinpour, Tetrahedron Lett., 2009, 50, 1533 CrossRef CAS; (g) A. M. Jacobine and G. H. Posnerm, J. Org. Chem., 2011, 76, 8121 CrossRef CAS PubMed; (h) O. A. Attanasi, L. De Crescentini, G. Favi, P. Filippone, G. Giorgi, F. Mantellini, G. Moscatelli and M. S. Behalo, Org. Lett., 2009, 11, 2265 CrossRef CAS PubMed; (i) I. Yavari, N. Hosseini, L. Moradi and A. Mirzaei, Tetrahedron Lett., 2008, 49, 4239 CrossRef CAS.
- (a) E. Lieber and R. Orlowski, J. Org. Chem., 1957, 22, 88 CrossRef CAS; (b) A. N. Vasiliev and A. D. Polackov, Molecules, 2000, 1014 CrossRef CAS; (c) B. Guo, Z. Ge, T. Chang and R. Li, Synth. Commun., 2001, 31, 3021 CrossRef CAS; (d) C. M. Buess, J. Am. Chem. Soc., 1955, 77, 6613 CrossRef CAS; (e) D. L. Fox, J. T. Ruxer, J. M. Oliver, K. L. Alford and R. N. Salvatore, Tetrahedron Lett., 2004, 45, 401 CrossRef CAS; (f) R. N. Salvatore, J. A. Ledger and K. W. Jung, Tetrahedron Lett., 2001, 42, 6023 CrossRef CAS; (g) R. N. Salvatore, S. Sahab and K. W. Jung, Tetrahedron Lett., 2001, 42, 2055 CrossRef CAS; (h) R. N. Salvatore, S. I. Shin, A. S. Nagle and K. W. Jung, J. Org. Chem., 2001, 66, 1035 CrossRef CAS PubMed; (i) W. Mc Ghee, D. Riley, K. Christ, Y. Pan and B. Parnas, J. Org. Chem., 1995, 60, 2820 CrossRef CAS.
- N. Kreutzkamp, H. Y. Oei and H. Peschel, Arch. Pharm. Ber. Dtsch. Pharm. Ges., 1971, 304, 649 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data and copies of 1H and 13C NMR spectra, IR spectra, TGA and DSC analysis of selected compounds, and GPC analysis for P5. See DOI: 10.1039/c6ra15616a |
| This journal is © The Royal Society of Chemistry 2016 |