A choline hydroxide catalyzed synthesis of 2,3-dihydroquinazolin-4(1H)-ones in an aqueous medium†
Abstract
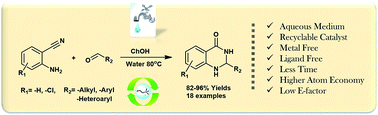
A simple, metal and ligand-free protocol for the synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives using choline hydroxide (ChOH) as an effective catalyst in an aqueous medium has been developed. The good to high yield of the desired product is obtained, with a low environmental factor and high atom economy. ChOH was found to be recyclable up to six consecutive runs without significant loss in its activity. The use of water as an environmentally benign solvent, ease of work up and efficient recyclability of the catalyst makes this process more advantageous and greener.

 Please wait while we load your content...
Please wait while we load your content...