Design and assembly of an aqueous red CdTe QD-LED: major factors to fabricate aqueous QD-LEDs†
Jinhua Dua,
Shuhong Xu*a,
Chunlei Wanga,
Jiangyong Panb,
Jing Chenb,
Li Zhua,
Changgui Lva and
Yiping Cui*a
aAdvanced Photonics Center, Southeast University, Nanjing 210096, Jiangsu, China. E-mail: xush@seu.edu.cn; cyp@seu.edu.cn
bDisplay Center, Southeast University, Nanjing 210096, Jiangsu, China
First published on 1st August 2016
Abstract
The first aqueous red CdTe QD-LED was fabricated with the structure of ITO/PEDOT:PSS/PVK/CdTe QDs/ZnO/Al. The device has been considered to be an advanced aqueous QD-LED, which demonstrated a turn-on voltage of about 5 V. Further, its current density and brightness would also reach 92 mA cm−2 and 58 cd m−2 at the voltage of 10 V. Moreover, we found that there were two essential factors for fabricating an aqueous QD-LED successfully: (1) adding wet agent to the QD solution, (2) removing the composite ions remaining in the QD solution. Meanwhile, the EL intensity of the aqueous QD-LEDs will perform better if the solution of QDs is acidic. These results offer a promising approach to the further development of aqueous QD-LEDs.
Introduction
The most interesting aspect of quantum dots (QDs) lies in the fact that they have several optical merits in terms of a narrow, tunable, and symmetric emission spectrum and outstanding photochemical stability, compared with organic dyes. Therefore, QDs have many applications in light-emitting diodes (LEDs),1,2 photovoltaic devices,3,4 photodetectors,5 and bio-labeling.6 Quantum dot light emitting devices (QD-LEDs) are a new generation of LEDs, which have attracted much attention in recent years, possessing unique properties of highly saturated emission, narrow emission with small full width at half maxima (FWHM), solution processability, and compatibility with flexible substrates.7 At present, the emitting layers of QD-LEDs are almost entirely QDs synthesized in organic solution via an organometallic route. Compared with organometallic routes, aqueous routes have many advantages. Water is environmentally-friendly and is able to dramatically decrease the synthesis cost. QDs prepared by aqueous routes have good biocompatibility, low synthesis temperature below 100 °C, high synthesis reproducibility, and the ability for scaling up synthesis. All these advantages make the aqueous route a better industrial and commercial prospect.8 In addition, many new luminescent materials are hydrophilic, such as carbon dots.9 Therefore, exploring the essential factors of fabricating aqueous QD-LEDs successfully is of great significance.Initial stage, this work tried to fabricate aqueous QD-LEDs in accordance with the traditional structure and method of organometallic QD-LEDs, but failed. In order to explore essential factors of fabricating aqueous QD-LEDs successfully, two different research directions depending on the differences between organometallic QDs and aqueous QDs were summarized. Firstly, in views of the incompatibility of water and organic solution, we suspected that aqueous QDs solution could not successfully spin coating onto the holes transport layer (HTL) whose solvent is organic solution. X. W. Sun etc. have confirmed this conjecture.10 They centrifuged and re-dispersed hydrophilic CdS QDs in a deionized (DI) water/Triton X-100 mixed solution with volume ratio of 2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Triton X-100 was used to reduce the surface tension of water and improve the quality of the hydrophilic QDs layer formed on top of the hydrophobic PVK substrate. Secondly, taking into account the preparation method of the aqueous QDs, we considered that acidity or alkalinity of the aqueous QDs solution and the composite ions remaining in the solution may be the essential factors of fabricating aqueous QD-LEDs successfully.
1. Triton X-100 was used to reduce the surface tension of water and improve the quality of the hydrophilic QDs layer formed on top of the hydrophobic PVK substrate. Secondly, taking into account the preparation method of the aqueous QDs, we considered that acidity or alkalinity of the aqueous QDs solution and the composite ions remaining in the solution may be the essential factors of fabricating aqueous QD-LEDs successfully.
Based on the above, our group set aqueous red CdTe QDs solution as an example to fabricate aqueous QD-LED with structure of ITO/PEDOT:PSS/PVK/CdTe QDs/ZnO/Al. The aqueous red CdTe QD-LED emitted successfully after CdTe solution being purified and added Triton X-100. The device demonstrated a turn-on voltage about 5 V. The current density and brightness were 92 mA cm−2 and 58 cd m−2 at the voltage of 10 V. It is considered to be an advanced aqueous QD-LED in the world. What's more, experiments showed that acidity or alkalinity of the QDs solution was not the essential factor to fabricate an aqueous QD-LED, but complex ions remaining in the solution would prevent the device from emitting light by disrupting the electron–hole recombination in the QD layer. In conclusion, adding wet agent to aqueous QDs solution and removing complex ions remaining in aqueous QDs solution are two essential processes of aqueous QD-LED emitting.
Experimental section
Materials
All materials used in this work are analytical reagents. Cadmium chloride (CdCl2) (98%), and sodium borohydride (NaBH4, 98%) were purchased from Aladdin. Poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (PEDOT:PSS) was purchased from Sigma-Aldrich. 3-Mercaptopropionic acid (MPA, 99+%), poly(N-vinylcarbazole) (PVK, average MW 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and tellurium (Te, 99.8%) powder were purchased from J&K. Ethanol, iso-propyl, acetone, ethyl acetate, dimethyl sulfoxide (DMSO), toluene, tetramethylammonium hydroxide (TMAH) were purchased from Sinopharm Chemical Reagent Co., Ltd. Zinc acetate dehydrate (99+%) was purchased from Shantou Longxi Chemical Reagent Co., Ltd. ITO Coated Glass was purchased from Zhuhai Kaivo Optoelectronic Technology Co., Ltd. Sodium hydrogen telluride (NaHTe) solution was prepared by using Te powder and NaBH4 in accordance with the reference methods.11,12 Colloidal ZnO nanocrystals were synthesized by a low-temperature solution-precipitation method with some modifications in accordance with Peng XG's method.2
000) and tellurium (Te, 99.8%) powder were purchased from J&K. Ethanol, iso-propyl, acetone, ethyl acetate, dimethyl sulfoxide (DMSO), toluene, tetramethylammonium hydroxide (TMAH) were purchased from Sinopharm Chemical Reagent Co., Ltd. Zinc acetate dehydrate (99+%) was purchased from Shantou Longxi Chemical Reagent Co., Ltd. ITO Coated Glass was purchased from Zhuhai Kaivo Optoelectronic Technology Co., Ltd. Sodium hydrogen telluride (NaHTe) solution was prepared by using Te powder and NaBH4 in accordance with the reference methods.11,12 Colloidal ZnO nanocrystals were synthesized by a low-temperature solution-precipitation method with some modifications in accordance with Peng XG's method.2
Synthesis of aqueous red CdTe QDs
CdTe QDs were prepared according to a previous method.11–13 Typically, freshly prepared NaHTe solution was injected into the solutions of CdCl2 and MPA after being degassed with nitrogen gas for 30 min at pH 9.0. To obtain CdTe QDs, the crude solution was refluxed at 100 °C and maintained for specific time and then cooled to room temperature in the open air. The concentration of Cd2+ was 4 × 10−3 mol L−1, and the feed molar ratio of Cd2+/MPA/NaHTe was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.4
2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2. The PL quantum efficiency (QY) of red CdTe QDs is about 45%. It was calculated by comparison to that of rhodamine 6G, which possessed known QY of 0.95.
0.2. The PL quantum efficiency (QY) of red CdTe QDs is about 45%. It was calculated by comparison to that of rhodamine 6G, which possessed known QY of 0.95.
Fabrication of aqueous red CdTe QD-LED
ITO glass substrate with a sheet resistance of about 7 V per square was cleaned by successive ultrasonic treatments in acetone, iso-propanol and DI water for 15 min, respectively. PEDOT:PSS as hole injection layer (HIL) was spin-coated onto ITO glass (2000 rpm, 40 s), followed by baking at 150 °C for 15 min. Thereafter, PVK as HTL was spin-coated at 1000 rpm for 40 s, followed by annealing at 120 °C for 35 min. The emissive layer of aqueous red CdTe QDs was centrifuged and re-dispersed in a DI water/Triton X-100 solution with volume ratio of 2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Simultaneously, the concentration of Cd2+ was concentrated to 3.5 × 10−2 mol L−1. After that the new aqueous red CdTe QDs solution was spin-coated on HTL (1000 rpm, 40 s) and annealed at 60 °C for 35 min. Then a colloidal ZnO nanocrystals solution was deposited on the QDs layer (1000 rpm, 40 s) and annealed at 60 °C for 30 min. Finally, a 100 nm aluminum (Al) electrode was thermally evaporated under high vacuum (431
1. Simultaneously, the concentration of Cd2+ was concentrated to 3.5 × 10−2 mol L−1. After that the new aqueous red CdTe QDs solution was spin-coated on HTL (1000 rpm, 40 s) and annealed at 60 °C for 35 min. Then a colloidal ZnO nanocrystals solution was deposited on the QDs layer (1000 rpm, 40 s) and annealed at 60 °C for 30 min. Finally, a 100 nm aluminum (Al) electrode was thermally evaporated under high vacuum (431![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 026 Torr) on top of ZnO nanocrystals, followed by post-annealing at 100 °C for 20 min. The active area of the devices was defined by a shadow mask of 16 mm2. Cross-sectional scanning electron microscope image of the device is shown in ESI Fig. S1.†
026 Torr) on top of ZnO nanocrystals, followed by post-annealing at 100 °C for 20 min. The active area of the devices was defined by a shadow mask of 16 mm2. Cross-sectional scanning electron microscope image of the device is shown in ESI Fig. S1.†
Device characterization
PL (photoluminescence) measurements were performed with a Shimadzu RF-5301 PC spectrofluorimeter. The excitation wavelength was 400 nm with a 10/10 nm slit. The current density–voltage characteristics were measured by Keithley-2400 source-meter, and electroluminescence (EL) spectra were recorded with an Ocean Optics Maya 2000-PRO spectrometer. The brightness was calibrated using a Minolta luminance meter (LS-100). Cross-sectional scanning electron microscope image was recorded by a Quanta 200 FEI with an acceleration voltage of 10 kV.Results and discussion
Aqueous red CdTe QD-LED
The schematics of our device structure and corresponding energy level diagram are shown in Fig. 1(a) and (b).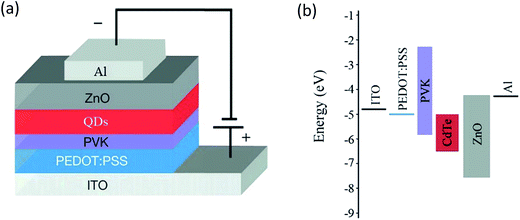 | ||
| Fig. 1 (a) A schematic of the device structure and (b) the corresponding energy band diagram of an aqueous red CdTe QD-LED. | ||
The device is constituted by using a patterned ITO as the anode, a PEDOT:PSS as the HIL, a PVK layer as the HTL, an aqueous red CdTe QD layer as the emissive layer, a ZnO layer as the electron transfer layer (ETL), and a 100 nm Al layer as the cathode. The QD-LED structure is designed to achieve efficient electrons and holes injection from the electrodes to the QD layer. Meanwhile, it is effectively blocking electrons and holes that pass through the QDs layer in terms of the energy levels of the constituent layers (Fig. 1(b)). A small injection step of 0.7 eV exists for the injection of electrons from Al to the QD layer since the ZnO has an electron affinity of 4.3 eV, similar to the work function of Al (4.3 eV). The small barrier between the highest occupied molecular orbital (HOMO) of PEDOT:PSS and PVK allow facile injection of holes from ITO to the QD layer. What's more, the high lowest unoccupied molecular orbital (LUMO) of PVK and low valence band (VB) of ZnO can effectively block injected electrons and holes respectively, leading to the confinement of the charges within the QDs layer.
Aqueous red CdTe QD-LED is fabricated successfully after CdTe solution being concentrated to 0.035 mol L−1, purified and added a certain percentage of Triton X-100 (DI water/Triton X-100 solution with volume ratio of 2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), as shown in Fig. 2. Fig. 3(a) demonstrates the EL spectrum of this QD-LED device, showing an emission peak centered at 698 nm. For comparison, the PL spectrum (red line) of QDs was also shown in Fig. 3(a). The very little changes between PL and EL spectrum indicate that the device emission is due entirely from QDs. The current versus voltage characteristic of the fabricated QD-LED is represented in Fig. 3(b). The device represents an EL turn-on at the forward voltage about 5 V. The current density and brightness of the QD-LED will also reach to 92 mA cm−2 and 58 cd m−2 at the voltage of 10 V. At the same time, it is considered to be an advanced aqueous QD-LED in the world similar to the only another aqueous QD-LED (hydrophilic CdS QD-LED).10
1), as shown in Fig. 2. Fig. 3(a) demonstrates the EL spectrum of this QD-LED device, showing an emission peak centered at 698 nm. For comparison, the PL spectrum (red line) of QDs was also shown in Fig. 3(a). The very little changes between PL and EL spectrum indicate that the device emission is due entirely from QDs. The current versus voltage characteristic of the fabricated QD-LED is represented in Fig. 3(b). The device represents an EL turn-on at the forward voltage about 5 V. The current density and brightness of the QD-LED will also reach to 92 mA cm−2 and 58 cd m−2 at the voltage of 10 V. At the same time, it is considered to be an advanced aqueous QD-LED in the world similar to the only another aqueous QD-LED (hydrophilic CdS QD-LED).10
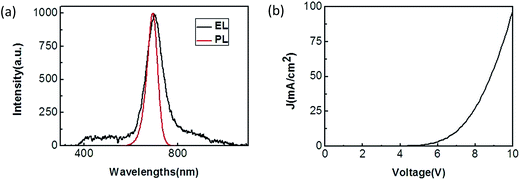 | ||
| Fig. 3 (a) EL spectrum (black line) of the QD-LED device at the driving voltage of 10 V and PL spectrum (red line) of QDs. (b) Current density versus voltage characteristics for the QD-LED. | ||
The influence of the composite ions Cd–MPA remaining in the aqueous QDs solution on the luminescence performance of aqueous red CdTe QD-LED
In order to explore the influence of the composite ions Cd–MPA remaining in the aqueous QDs solution on the luminescence performance of QD-LED, LED sample A and LED sample B were fabricated to make some comparison. Except the emitting layer (QDs) is different, other fabrication and testing conditions of sample A and sample B are exactly the same. The QDs used in sample A is the standard QDs, which is concentrated to 0.035 mol L−1, purified and added a certain percentage of Triton X-100 (DI water/Triton X-100 solution with volume ratio of 2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Correspondingly, the QDs solution used in sample B is added additional composite ions Cd–MPA after being done the same treating just like the QDs solution of sample A. The experimental step in detail is shown in ESI† “The method of preparing QDs solution used in sample B”. After being coated QDs, sample B looked spotted while sample A looked more transparent, as shown in Fig. 4(a). When a forward voltage was applied, sample A emitted uniform and stable red light, but sample B was not lighted. Additionally, as shown in Fig. 4(b), the current density versus voltage characteristics for sample B was a straight line, which showed that the device did not form a LED at all. The reason for sample B failed could be related that negative charges of Cd–MPA prevent electrons and holes recombining in the QD layer. Therefore, removing complex ions remaining in aqueous QDs solution is essential factor for fabricating aqueous QD-LEDs successfully.
1). Correspondingly, the QDs solution used in sample B is added additional composite ions Cd–MPA after being done the same treating just like the QDs solution of sample A. The experimental step in detail is shown in ESI† “The method of preparing QDs solution used in sample B”. After being coated QDs, sample B looked spotted while sample A looked more transparent, as shown in Fig. 4(a). When a forward voltage was applied, sample A emitted uniform and stable red light, but sample B was not lighted. Additionally, as shown in Fig. 4(b), the current density versus voltage characteristics for sample B was a straight line, which showed that the device did not form a LED at all. The reason for sample B failed could be related that negative charges of Cd–MPA prevent electrons and holes recombining in the QD layer. Therefore, removing complex ions remaining in aqueous QDs solution is essential factor for fabricating aqueous QD-LEDs successfully.
 | ||
| Fig. 4 (a) The picture of sample A (left) and sample B (right), (b) current versus voltage characteristic of sample B. | ||
The influence of acidity or alkalinity of the aqueous QDs solution on the luminescence performance of aqueous red CdTe QD-LED
We set up three groups of sample to explore the influence of acidity or alkalinity of the aqueous QDs solution on the luminescence performance of QD-LED. Except their pH values were different, the QDs solution used in sample I, II, III were all concentrated to 0.035 mol L−1, purified and added a certain percentage of Triton X-100 (DI water/Triton X-100 solution with volume ratio of 2000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The pH values of QDs used in sample I, II, III were 4.0, 6.8, 10.0, respectively. After being applied a forward voltage, sample I, II, III all emit uniform and stable red light. Their current versus voltage characteristics were shown in Fig. 5(a). As can be seen from the figure, their turn-on voltages were about 3 V, 5 V, 5 V, respectively. And their current density could reach to 167 mA cm−2, 92 mA cm−2, 58 mA cm−2 at the voltage of 10 V. Fig. 5(b) demonstrated the EL spectrums of sample I, II, III at the voltage of 10 V. In Fig. 5(b), it could be seen that the EL intensity decreased with the pH rising from 4.0 to 10.0, but the peak position and shape of EL spectrums remained unchanged. Therefore, we conclude that the acidity or alkalinity of the aqueous QDs solution is not essential factor for fabricating aqueous QD-LEDs successfully. The reason is the following two points. First, the Triton X-100 used in our QDs solutions may protect QDs from quenching when their pH value is 4.0 or 10.0. Second, QDs in different pH value solution are immediately being spin-coated and annealed. The pH effect does not have chance to change PL of QDs as soon as QDs solutions became QDs layer (Fig. 5(c)). In a state of layer, the fluorescence of QDs would not influence by pH value of solution. For the above reasons, our QD-LEDs could emit successfully whether the QDs solution is acidic or alkaline. Moreover, acidic solvent of QDs are beneficial to the luminescence of aqueous QD-LED. The reason is that acidic solvent could lead to electrons and holes recombining better in the QDs layer by removing the Cd–MPA attached to the surface of the QDs.
1). The pH values of QDs used in sample I, II, III were 4.0, 6.8, 10.0, respectively. After being applied a forward voltage, sample I, II, III all emit uniform and stable red light. Their current versus voltage characteristics were shown in Fig. 5(a). As can be seen from the figure, their turn-on voltages were about 3 V, 5 V, 5 V, respectively. And their current density could reach to 167 mA cm−2, 92 mA cm−2, 58 mA cm−2 at the voltage of 10 V. Fig. 5(b) demonstrated the EL spectrums of sample I, II, III at the voltage of 10 V. In Fig. 5(b), it could be seen that the EL intensity decreased with the pH rising from 4.0 to 10.0, but the peak position and shape of EL spectrums remained unchanged. Therefore, we conclude that the acidity or alkalinity of the aqueous QDs solution is not essential factor for fabricating aqueous QD-LEDs successfully. The reason is the following two points. First, the Triton X-100 used in our QDs solutions may protect QDs from quenching when their pH value is 4.0 or 10.0. Second, QDs in different pH value solution are immediately being spin-coated and annealed. The pH effect does not have chance to change PL of QDs as soon as QDs solutions became QDs layer (Fig. 5(c)). In a state of layer, the fluorescence of QDs would not influence by pH value of solution. For the above reasons, our QD-LEDs could emit successfully whether the QDs solution is acidic or alkaline. Moreover, acidic solvent of QDs are beneficial to the luminescence of aqueous QD-LED. The reason is that acidic solvent could lead to electrons and holes recombining better in the QDs layer by removing the Cd–MPA attached to the surface of the QDs.
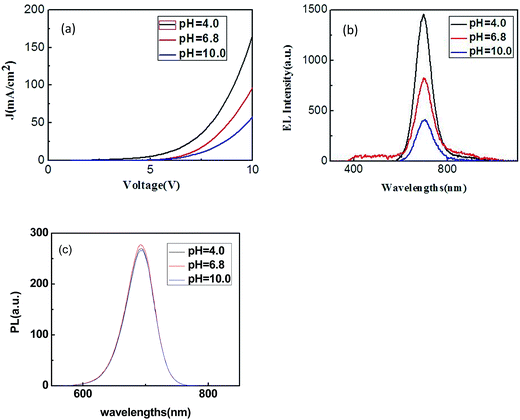 | ||
| Fig. 5 (a) Current versus voltage characteristic of sample I, II, III; (b) EL spectrums of sample I, II, III at the driving voltage of 10 V; (c) PL spectrums of sample I, II, III. | ||
Conclusions
In summary, the first and advanced aqueous red CdTe QD-LED has been fabricated. The device represents an EL turn-on at the forward voltage about 5 V, and its current density and brightness reach to 92 mA cm−2 and 58 cd m−2 at the voltage of 10 V. Meanwhile, we found that there were two essential processes to fabricate an aqueous QD-LED successfully: adding wet agent to QDs solution and removing the complex ions remaining in the QDs solution. Adding wet agent can reduce the surface tension of water and improve the quality of the hydrophilic QDs layer formed on top of the hydrophobic HTL substrate. Removing complex ions would prevent them from disrupting the electron–hole recombination in the QD layer. What's more, the acidity or alkalinity of the aqueous QDs solution is not essential factor for fabricating aqueous QD-LEDs successfully, but acidic solvent of QDs are beneficial to the luminescence of aqueous QD-LED because that acidic solvent can lead to electrons and holes recombining better in the QDs layer by removing the Cd–MPA attached to the surface of the QDs. Our work would offer a promising approach for other aqueous QDs applied in QD-LEDs.Acknowledgements
This work is supported by the National Key Basic Research Program of China (Grant No. 2015CB352002), National Nature Science Foundation of China (Grant No. 11274062, 61475034, 61378045, 21403034), the Fundamental Research Funds for the Central Universities (No. 2242014R30006), the Natural Science Foundation of Jiangsu Province Youth Fund (Grant No. BK20140650) and the Natural Science Foundation of Jiangsu Province (Grant No. BK20131297).References
- V. L. Colvin, M. C. Schlamp and A. P. Alivisatos, Nature, 1994, 370, 354–357 CrossRef CAS.
- X. Dai, Z. Zhang, Y. Jin, Y. Niu, H. Cao, X. Liang, L. Chen, J. Wang and X. Peng, Nature, 2014, 515, 96–99 CrossRef CAS PubMed.
- I. Gur, N. A. Fromer, M. L. Geier and A. P. Alivisatos, Science, 2005, 310, 462–465 CrossRef CAS PubMed.
- G. Lan, Z. Yang, Y. Lin, Z. Lin, H. Liao and H. Chang, J. Mater. Chem., 2009, 19, 2349–2355 RSC.
- G. Konstantatos, I. Howard, A. Fischer, S. Hoogland, J. Clifford, E. Klem, L. Levina and E. H. Sargent, Nature, 2006, 442, 180–183 CrossRef CAS PubMed.
- I. L. Medintz, A. R. Clapp, H. Mattoussi, E. R. Goldman, B. Fisher and J. M. Mauro, Nat. Mater., 2003, 2, 630–638 CrossRef CAS PubMed.
- J. Chen, D. Zhao, C. Li, F. Xu, W. Lei, L. Sun, A. Nathan and X. Sun, Sci. Rep., 2014, 4, 4085–4090 Search PubMed.
- Z. Hu, S. Xu, X. Xu, Z. Wang, Z. Wang, C. Wang and Y. Cui, Sci. Rep., 2015, 5, 14817–14825 CrossRef CAS PubMed.
- Y. P. Sun, B. Zhou, Y. Lin, W. Wang, K. A. Fernando, P. Pathak, M. J. Meziani, B. A. Harruff, X. Wang, H. Wang, P. G. Luo, H. Yang, M. E. Kose, B. Chen, L. M. Veca and S. Y. Xie, J. Am. Chem. Soc., 2006, 128, 7756–7757 CrossRef CAS PubMed.
- M. Molaei, M. Marandi, E. Saievar-Iranizad, N. Taghavinia, B. Liu, H. D. Sun and X. W. Sun, J. Lumin., 2012, 132, 467–473 CrossRef CAS.
- H. Zhang, Z. Zhou, Y. Bai and M. Gao, J. Phys. Chem. B, 2003, 107, 8–13 CrossRef CAS.
- C. Wang, H. Zhang, S. Xu, N. Lv, Y. Liu, M. Li, H. Sun, J. Zhang and B. Yang, J. Phys. Chem. C, 2008, 113, 827–833 Search PubMed.
- C. Wang, M. Fang, J. Han, H. Zhang, Y. Cui and B. Yang, J. Phys. Chem. C, 2009, 113, 19445–19451 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra15558h |
| This journal is © The Royal Society of Chemistry 2016 |

