Alginic acid/graphene oxide hydrogel film coated functional cotton fabric for controlled release of matrine and oxymatrine†
Abstract
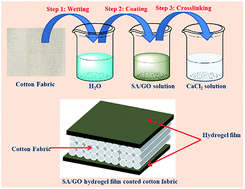
The present study describes the fabrication of a functional cotton fabric in which an alginic acid/graphene oxide hydrogel layer was coated on the surface of cotton fabric. The functional fabric was then used to absorb and release two classic Chinese traditional drugs, matrine and oxymatrine, to investigate the controlled release capability of the functional fabric. The obtained results indicated that the prepared functional fabric has a sandwich structure. This structure significantly enhanced the absorbing capability of the cotton fabric for both water and the two drugs. Moreover, with the incorporation of the graphene oxide, the functional cotton fabric could steadily release the absorbed drugs and prevent burst release. It was also found that the release speed of the two drugs could be controlled through tuning the environmental temperature. The prepared functional cotton fabric has large application potentials in many external medical care fields.

 Please wait while we load your content...
Please wait while we load your content...