Improved dispersibility of nano-graphene oxide by amphiphilic polymer coatings for biomedical applications
Abstract
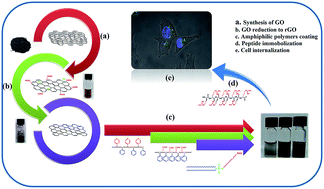
The poor dispersibility and stability of graphene and its derivatives in aqueous media is an influential limitation in the biological applications of graphene based nanomaterials. The aim of this study is to improve the solubility and dispersibility of reduced graphene oxide (rGO) in cell culture media and serum using three amphiphilic polymers (polystyrene-co-polyacrylic acid (PS-PAA), polystyrene-co-poly4-vinylpyridine (PS-P4V) and phospholipid-polyethylene glycol (PL-PEG)) as first layer coating materials. A cationic cell penetrating peptide, octaarginine (R8), is incorporated as a second layer on the selected formulation to enhance the cell internalization capability of the stabilized rGO. Chemical, physical and biological properties of the double-layer coated (DLC)-rGO are fully characterized. The cellular uptake of fluorescein isothiocyanate (FITC)-labeled DLC-rGO is evaluated using confocal microscopy. None of the polymer-functionalized rGO samples show cell toxicity. While the amount of immobilized PL-PEG on the rGO surface is less than PS-PAA and PS-P4V, much higher dispersibility is observed than with other copolymers. Furthermore, the R8 coated rGO-PL-PEG shows great cell internalization ability after 4 h incubation with MCF-7 cells. It is concluded that DLC-rGO could have great potential to be used as a nanocarrier in the biomolecular delivery of biological materials such as drugs and genes.

 Please wait while we load your content...
Please wait while we load your content...